��Ŀ����
����Ŀ��ʵ������ 500��ʱ�����������������������[(NH4)2Fe(SO4)2]���ֽ���ȫ��ȷ���ֽ����ɷֵ�װ����ͼ��ʾ (��֪�ֽ�Ĺ����������� FeO��Fe2O3 �� Fe3O4�������������� NH3��N2��H2O��SO3 �� SO2)������˵���� ȷ����( )
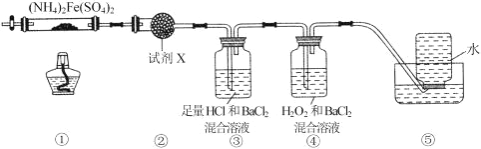
A. ȡ���й����������ϡ���ᷴӦ���μ� KSCN����Һ��죬�������һ��Ϊ Fe2O3
B. װ�â����ڼ���ֽ�������Ƿ���ˮ�������ɣ��Լ� X ���ѡ�ü�ʯ��
C. װ�â����ڼ���ֽ�������Ƿ��� SO3 �������ɲ���ȥ SO3 �� NH3
D. װ�â����ڼ���ֽ�������Ƿ��� SO2 �������ɣ�װ�â������ռ����ɵ� NH3 �� N2
���𰸡�C
��������
A��Fe3O4�к����������������Ӻ�һ�����������ӣ�ȡ���й����������ϡ���ᷴӦ���μ� KSCN����Һ��죬����������Ϊ Fe2O3��Fe3O4�������߾��У���A����
B��װ�â����ڼ���ֽ�������Ƿ���ˮ�������ɣ��Լ� X ���ѡ����ˮ����ͭ����B����
C��װ�â������������ɫ���������ɫ����Ϊ���ᱵ����֤������ SO3 ���壬�����Գ�ȥ SO3 ��װ�â��������ᣬ��˿��Գ�ȥ NH3����C��ȷ��
D��NH3 ��������ˮ����������ˮ���ռ����ڰ���������װ��ʱ�Ѿ�����ȥ����ˢ������ռ����ɵ�N2����D����
����������������ȷ��ΪC��

����Ŀ����ͳ�����β����������¡�����ʴ��ǿ�ȸߵ��������ܣ����㷺Ӧ���ڸ��ֹ�ҵ����ѧ�о����ճ������С�ij�����β��ϵ���Ҫ�ɷ�Ϊ�����ơ��������裬������һ������������þ�������ij�о�С������������̲ⶨ�ù����β����иƵĺ���������ͼ��ʾ����
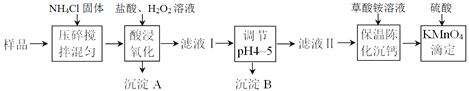
����������������������ʽ����ʱ��Һ��pH���±�:(��������Ũ����0.1mol��L-1��)
������ | Fe(OH)3 | Fe(OH)2 | Ca(OH)2 | Al(OH)3 | Mg(OH)2 |
��ʼ����pH | 2.7 | 7.6 | 12.3 | 4.0 | 8.0 |
��ȫ����pH | 3.7 | 9.6 | 14.3 | 5.2 | 12.4 |
�ش���������:
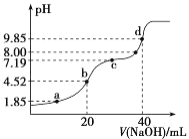
��1���������˫��ˮ��������Ӧ�����ӷ���ʽΪ_________________��Ϊ��߸ƵĽ����ʣ����Բ�ȡ�Ĵ�ʩ��________________________������дһ�֣�
��2������B�ijɷ�Ϊ_____________��������Һ���pHΪ4~5����������ʿ�����_________��
A. CaCO3 B. Ca(OH)2 C. ��ˮ D. MgO
��3������Һ���м������������Һ����ˮԡ�ϱ��³»�2Сʱ����ȴ�����£����ˡ�ϴ�ӳ��������õ���CaC2O4�����ܽ��������У���ϡ�����250mL��Һ������Һ����ȡ25.00 mL��Һ����ƿ�У���KMnO4����Һ�ζ����ζ�ʱ������Ӧ�����ӷ���ʽΪ___________________________����ʵ������ȡ��Ʒ����Ϊ4.00 g ��KMnO4����ҺŨ��Ϊ0.0500 mol/L��ƽ�еζ�3����ȥKMnO4����Һ���ƽ��ֵΪ36.00 mL����ù�������Ʒ�иƵ���������Ϊ_________��
��4���ڱ��³��ƻ��ڣ�����Ʒ��þ�ĺ������ߣ��ᵼ�����ղⶨ��������ϴ����Դ������ϼ��㣬�Ӳ������Һ����ʱ����Һ��þ���ӵ�Ũ����߲��ܳ���____mol/L������֪��Ksp(CaC2O4) = 4.00��10-9��Ksp(MgC2O4) = 4.83��10-6��