��Ŀ����
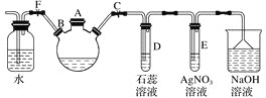
����Ŀ������ͼװ����ʾ��C��D��E��F��X��Y���Ƕ��Ե缫���ס�������Һ�������Ũ�ȶ���ͬ������ͨ��ǰ����Һ������䣩��A��BΪ���ֱ����Դ����������ֱ����Դ��ͨ��F�������ʺ�ɫ�� ��ش�

��1��B���ǵ�Դ��__________����C���ĵ缫��ӦʽΪ____________________________________��һ��ʱ�����X����������ɫ��______________�����������ߡ���dz����
��2�����ס���װ���е�C��D��E��F�缫��ֻ��һ�ֵ�������ʱ����Ӧ���ʵ����ʵ���֮��Ϊ ______________��
��3�����ñ�װ�ø�ͭ����������HӦ����______________ ������ͭ�����������������Һ��___________ ��Һ����������Һ��pH��13ʱ����ʱ����Һ���Ϊ500mL�������жƼ���������������Ϊ________ g��������Һ��pH _____________�����������������С����������������
��4�������ձ��������������ͭ����֪���ǰ���缫������ͬ�������ɺ�����ȡ����ϴ������ɡ����������ֶ����������5.12g������ʱ��·��ͨ���ĵ���Ϊ_______mol
���𰸡���4OH-�� 4 e����O2+2H2O��dz1��2��2��2ͭAgNO35.4��С0.08
��������
C��D��E��F��X��Y���Ƕ��Ե缫���ס�������Һ�������Ũ�ȶ���ͬ(����ͨ��ǰ����Һ�������)��A��BΪ���ֱ����Դ����������ֱ����Դ��ͨ����F�������ʺ�ɫ��˵��F�缫�����м���������F�缫�������ӷŵ�����������Ϊ����������C��E��G��X��������D��F��H��Y�����������������ĵ缫A�����������������ĵ缫B�Ǹ������ݴ˷�����
(1)������Ϸ��������Ե缫��ⱥ��NaCl��Һ���ܷ�Ӧ����ʽΪ��2NaCl+2H2O![]() 2NaOH+H2��+Cl2����F���Ժ�ɫ��˵��F�������������缫��Ӧ2H2O+2e-=2OH-+ H2������B�缫�ǵ�Դ�ĸ�����A�ǵ�Դ��������C���Դ������������C���������ö��Ե缫���CuSO4��Һʱ��ˮ�������OH-�������ŵ������������缫��ӦʽΪ4OH-�� 4 e����O2��+2H2O�����������������巢����Ӿ����Y�������������������������Ӵ�����ɣ���Y���ƶ�������X�缫��ɫ��dz��
2NaOH+H2��+Cl2����F���Ժ�ɫ��˵��F�������������缫��Ӧ2H2O+2e-=2OH-+ H2������B�缫�ǵ�Դ�ĸ�����A�ǵ�Դ��������C���Դ������������C���������ö��Ե缫���CuSO4��Һʱ��ˮ�������OH-�������ŵ������������缫��ӦʽΪ4OH-�� 4 e����O2��+2H2O�����������������巢����Ӿ����Y�������������������������Ӵ�����ɣ���Y���ƶ�������X�缫��ɫ��dz��
��ˣ�������ȷ����������4OH-�� 4 e����O2��+2H2O ����dz��
��2��C�缫��ӦΪ4OH-�� 4 e����O2��+2H2O��D�缫��ӦΪCu2++2e-=Cu��E�缫��ӦΪ2Cl��2e-=Cl2����F�缫��ӦΪ2H2O+2e-=2OH-+H2����һ��ʱ���ڣ��ĸ��缫ת�Ƶĵ�������ͬ�����Լס���װ�õ�C��D��E��F�缫���е������ɣ������ʵ���֮��Ϊ1:2:2:2��
��ˣ�������ȷ������1��2��2��2��
(3)��װ���ǵ�Ƴ����Ʋ�AgΪ�������Ƽ�CuΪ���������Һ����������Һ��G��������H������������G�ǶƲ�Ag��H�ǶƼ�Cu�����Һ����������Һ�������£�������Һ��pH=13����c(OH-)=0.1mol/L����Һ�����Ϊ500mL����n(OH-)=0.1mol/L��0.5L=0.05mol�����ݵ缫��Ӧʽ2H2O+2e-=2OH-+H2������֪ת�Ƶ��ӵ����ʵ���Ϊ0.05mol�����ݶ�����������Ӧʽ��Ag++e-=Ag����֪������������Ϊ0.05mol��108g/mol=5.4g����װ���Ƕ��Ե缫�������ͭ��Һ���ܷ�Ӧʽ2CuSO4+2H2O![]() 2Cu+2H2SO4+O2������Ӧ���������ᣬ���Լ�����Һ��pH��С��
2Cu+2H2SO4+O2������Ӧ���������ᣬ���Լ�����Һ��pH��С��
��ˣ�������ȷ������ͭ��AgNO3 ��5.4����С��
(4) ���ʱ�������Ͻ���ʧ���ӵ��½���������������������������������������������ƺ������������һ��Ϊ������������ͭ��������������������ͭ������=5.12g��![]() =2.56g���ɵ缫��ӦCu2++2e-=Cu֪��ת�Ƶ��ӵ����ʵ���=
=2.56g���ɵ缫��ӦCu2++2e-=Cu֪��ת�Ƶ��ӵ����ʵ���=![]() 0.08mol��
0.08mol��
��ˣ�������ȷ������0.08��