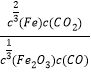��Ŀ����
����Ŀ����֪X��Y��Z��M��G��Q�����ֶ���������Ԫ�أ�ԭ��������������X��Z��Q�ĵ����ڳ����³���̬��Y��ԭ������������������Ӳ�����2����X��Mͬ���壬Z����̬�⻯������������������ˮ���ﷴӦ��G�ǵؿ��к�����ߵĽ���Ԫ�ء���ش��������⣺
(1)Q��Ԫ�ط���Ϊ______��Y��Z��M��G����Ԫ��ԭ�Ӱ뾶�ɴ�С��˳����(дԪ�ط���)_______��
(2)Y��Ԫ�����ڱ��е�λ��Ϊ_______________��Y����Ԫ���γɵĶ�Ԫ������ĵ���ʽΪ_________________________��
(3)����Ԫ�ص�����������Ӧ��ˮ����������ǿ����(д��ѧʽ)_____________��
(4)����Y��Ԫ��Z������������Ӧˮ�����Ũ��Һ������Ӧ�Ļ�ѧ����ʽΪ_________��
(5)Z��G��ɵĻ�����GZ�������������������Ԫ������ҵ����G�������Y���ʺ�Z�����ڸ������Ʊ�GZ������G���������Y���ʵ����ʵ���֮��Ϊ1��3����÷�Ӧ�Ļ�ѧ����ʽΪ__________________________��
���𰸡�Cl Na��Al��C��N �ڶ����ڵڢ�A�� ![]() HClO4 C+4HNO3(Ũ)
HClO4 C+4HNO3(Ũ)![]() CO2��+4NO2��+2H2O Al2O3+3C+N2
CO2��+4NO2��+2H2O Al2O3+3C+N2![]() 2AlN+3CO
2AlN+3CO
��������
G�ǵؿ��к�����ߵĽ���Ԫ�أ�GΪ��Ԫ�أ�Z����̬�⻯������������������ˮ���ﷴӦ��ZΪ��Ԫ�أ�X��Z��Q�ĵ����ڳ����³���̬��XΪ��Ԫ�أ�ZΪ��Ԫ�أ�QΪ��Ԫ�أ�Y��ԭ������������������Ӳ�����2����YΪ̼Ԫ�أ�X��Mͬ���壬MΪ��Ԫ�أ��ݴ˽��
�������Ϸ�������֪����XΪ�⣬YΪ̼��ZΪ����MΪ�ƣ�GΪ����QΪ�ȡ���
��1��QΪ��Ԫ�أ�Ԫ�ط���Cl��C��N��Na��Cl����Ԫ�أ�������Խ��ԭ�Ӱ뾶Խ��ͬһ���ڣ�ԭ������ԽС���뾶Խ������ԭ�ӵ�ԭ�Ӱ뾶�ɴ�С��˳���ǣ�Na��Al��C��N��
��2��YԪ��Ϊ̼���˵����6�����ڱ��е�λ��Ϊ�ڶ����ڵڢ�A�壻̼����Ԫ���γɵĶ�Ԫ������Ϊ����̼��Ϊ���ۻ��������ʽΪ![]() ��
��
��3��Ԫ�صķǽ�����Խǿ����Ӧ����������ˮ��������Ծ�Խǿ��XΪ�⣬YΪ̼��ZΪ����MΪ�ƣ�GΪ����QΪ�ȣ���Щ��Ԫ���У��ǽ�������ǿ��Ϊ��Ԫ�أ�����������Ӧ��ˮ����������ǿ����ѧʽΪHClO4��
��4������YΪ̼��Ԫ��Z������������Ӧˮ�����Ũ��ҺΪŨ���ᣬ̼��Ũ������������·�Ӧ���ɶ��������Ͷ�����̼����ѧ����ʽΪC+4HNO3(Ũ)![]() CO2��+4NO2��+2H2O��
CO2��+4NO2��+2H2O��
��5��ZΪ����GΪ������ɵĻ����ﵪ��������ҵ����G��������Ϊ��������Y����Ϊ̼��Z����Ϊ�������ڸ����·�Ӧ�Ʊ���������������������̼���ʵ����ʵ���֮��Ϊ1��3���÷�Ӧ�Ļ�ѧ����ʽΪAl2O3+3C+N2![]() 2AlN+3CO��
2AlN+3CO��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�