��Ŀ����
����Ŀ�������ģ�����������;�㷺����ʳƷ�п���Ϊ����ԭ�ϣ����������·����Ʊ����ᴿ��
���Ʊ�
���Թ�a���ȼ���3mLCH3CH2OH����ҡ����������2mLŨH2SO4�����ҡ�ȣ���ȴ���ټ���2mLCH3CO18OH����ֻ�Ϻ��Թ̶ܹ�������̨�ϣ����Թ�b�м���7mL����̼������Һ�����Ӻ�װ�á��þƾ��ƶ��Թ�a���ȣ����۲쵽�Թ�b������������ʱֹͣ���ȡ�

��1����װ����һ��������ָ��__________________________��
��2���Թ�a���������������Ļ�ѧ����ʽ����dz�18O��λ�ã�______________________________��
��3������ŨH2SO4��������______________________________��
II���ᴿ��

��1������1���õ�_____________�����������ƣ����ϲ�Һ��Ӹ�������_________�����������Ͽڡ����¿ڡ�����
��2���������������������ѡ����ʵĸ����__________��
a.�Ȼ��� b.��ʯ�� c.����ͭ
��3���Թ�b�л��Һ���������������ݲ������÷�Ӧ�Ļ�ѧ����ʽΪ_________________________��
���𰸡�b�е������뵽��Һ������CH3CO18OH+CH3CH2OH![]() CH3COOCH2CH3+H218O��������ˮ����Һ©���Ͽ�a2CH3COOH+Na2CO3��2CH3COONa+CO2��+H2O��������CH3CO18OH��ʾ��
CH3COOCH2CH3+H218O��������ˮ����Һ©���Ͽ�a2CH3COOH+Na2CO3��2CH3COONa+CO2��+H2O��������CH3CO18OH��ʾ��
��������
��1��������������ʱҪ��ֹ������
��2��������Ӧ�������ṩ�ǻ������ṩ��ԭ����
��3������������Ӧԭ���ж���
II����1������1�Ƿ�Һ�����ݷ�Һ�������
��2���������������ڼ�����Һ����ˮ�������
��3����������̼���Ʒ�Ӧ�������塣
��1���������ɵ����������к����Ҵ������ᣬ���߾���ˮ���ܣ�����װ���е��ܿڲ��ܲ�����Һ�У����װ�õĴ�����b�е������뵽��Һ�����¡�
��2��������Ӧ�������ṩ�ǻ������ṩ��ԭ�������Թ�a���������������Ļ�ѧ����ʽΪCH3CO18OH+CH3CH2OH![]() CH3COOCH2CH3+H218O��
CH3COOCH2CH3+H218O��
��3��������Ӧ�ǿ��淴Ӧ�������ŨH2SO4�������Ǵ�������ˮ����
II����1������1�Ƿ�Һ�����õ��������Ƿ�Һ©�����ϲ�Һ��Ӹ��������Ͽڷ��������
��2���������������ڼ�����Һ��������ˮ�ⷴӦ������ͭ�����������������ѡ���Ȼ��Ƹ���������������ѡa��
��3���Թ�b�л��Һ���������������ݲ�������������̼���������ᷴӦ�����ģ���÷�Ӧ�Ļ�ѧ����ʽΪ2CH3COOH+Na2CO3��2CH3COONa+CO2��+H2O��

����Ŀ����ˮ���Ȼ�����SnCl4���������л��ϳɵ��Ȼ�������ʵ���ҿ������ڵ������۵�231.9�棩��Cl2��Ӧ�Ʊ�SnCl4��װ����ͼ��ʾ���ش��������⣺
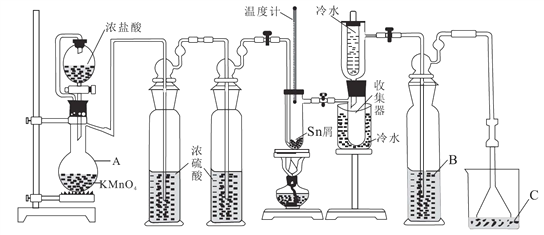
��֪����SnCl4�ڿ����м���ˮ������SnO2����H2O
��
���� | ��ɫ��״̬ | �۵�/�� | �е�/�� |
SnCl2 | ��ɫ���� | 246 | 652 |
SnCl4 | ��ɫҺ�� | ��33 | 114 |
��1������A��������_______�����з�����Ӧ�����ӷ���ʽ��____________��
��2����ȼ�ƾ���ǰ���ž�װ���еĿ���������ᷢ������Ӧ����ѧ����ʽ��______��_____��
��3���Լ� B��C����Ϊ_______���Լ�B��������______��
��4��Cl2 �����ķ�Ӧ������SnCl4��SnCl2��Ϊ��ֹ��Ʒ�д��� SnCl2���ɲ�ȡ�Ĵ�ʩ��_______��_______��
��5�����������ζ�������Ʒ��2��Sn��II���ĺ�����ȷ��ȡm g��Ʒ����ƿ�У�������ˮ�ܽ�������-KI��Һ��ָʾ������c mol��L��1 �����Һ�ζ����յ������ĵ����ҺV mL�������Ʒ��Sn��II���ĺ���_______���ú�c��m��V�Ĵ���ʽ��ʾ����