��Ŀ����
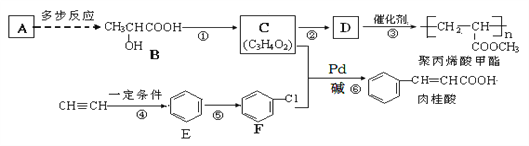
����Ŀ��������Ԫ��A��B��C��D��E��Ԫ�����ڱ��е�λ������ͼ��ʾ������BԪ����̼Ԫ�ء���ش���������:
��1��BԪ�������ڱ��е�λ�ã�_________________����д����ԭ�ӵĵ���ʽ��___________����ԭ�Ӻ������������ߵ��ܼ��ǣ�_______��
��2���Ƚ�A��C��DԪ�ؼ����Ӱ뾶�Ĵ�С��_______>_______>_______ (��������)��
��3��F��Dͬ���������ڣ���Ƚ�D���⻯���F���⻯���ȶ��Դ�С��_______ (�û�ѧʽ��ʾ)��
��4��E���ʳ���ʪ��ĵ��۵⻯����ֽ���顣��ѧ���Ե��۵⻯�ؽ�����ʵ��̽�����ڵ��۵⻯����Һ�У��μ���������������Һ�����̻ῴ����Һ����ɫ��������Ϊ_______������������ԭ��Ӧ֪ʶ�������������ͬ���������γɵ���ɫ��Һ�У��ٵμ�����������Һ��������ɫ����ʧ��������Ϊ_________________���ݴ˷�����I2��ClO-��SO42-������������ǿ��˳������Ϊ____________________��
���𰸡� �ڶ�����IVA ![]() 2P N3- O2- Al3+ H2O>H2S NaClO��KI����������I2 I2��Na2SO3��ԭ������I- SO42-< I2-
2P N3- O2- Al3+ H2O>H2S NaClO��KI����������I2 I2��Na2SO3��ԭ������I- SO42-< I2-
��������������Ԫ��A��B��C��D��E,BԪ���γɵĻ���������Ȼ���е��������,��BΪCԪ��,��Ԫ�������ڱ��е�λ�������Ƴ�CΪN,DΪO,AΪAl,EΪCl��
(1) BΪ̼Ԫ�أ��˵����Ϊ6�������ڱ��еڶ����ڵ�IVA��������������ĸ�������������ʽΪ![]() ����������Ų�Ϊ1s22s22p2����ԭ�Ӻ������������ߵ��ܼ���2P����ȷ�𰸣��ڶ�����IVA��
����������Ų�Ϊ1s22s22p2����ԭ�Ӻ������������ߵ��ܼ���2P����ȷ�𰸣��ڶ�����IVA�� ![]() �� 2P��
�� 2P��
(2)A��C��DԪ�ؼ�����:![]() ��
��![]() ��
��![]() ��������ͬ�ĵ����Ų�,��ԭ������������Ӱ뾶С,�������Ӱ뾶Ϊ
��������ͬ�ĵ����Ų�,��ԭ������������Ӱ뾶С,�������Ӱ뾶Ϊ![]() ����ȷ����. N3- �� O2- ��Al3+ ��
����ȷ����. N3- �� O2- ��Al3+ ��
��3��F��Dͬ����������,��FΪS,��ǽ�����![]() ,��O���⻯���S���⻯���ȶ���H2O>H2S ����ȷ���� H2O>H2S��
,��O���⻯���S���⻯���ȶ���H2O>H2S ����ȷ���� H2O>H2S��
��4��NaClO��Һ����ǿ�����ԣ��ܹ��ѵ���������Ϊ�ⵥ�ʣ�����������Һ�������������ƾ��л�ԭ�ԣ��ܹ��ѵԭΪ�����ӣ�����������Ϊ��������ӣ�������ɫ����ʧ���������Ӳ���ʹ������Һ�������������Ϸ�����֪��ClO-�����Դ��ڵ⣬��������Դ�����������ӣ�����I2��ClO-��SO42-������������ǿ��˳������ΪSO42-< I2<ClO-����ȷ�𰸣�NaClO��KI����������I2 �� I2��Na2SO3��ԭ������I- ��SO42-< I2<ClO-��

����Ŀ����ⷨ������ʱ����������������࣬����Ҫ�ɷ�ΪMnO2��Pb���������������������������ͼ�ǻ��յ��������������MnCO3�Ĺ������̡�

��֪��Al(OH)3��Mn(OH)2��MnCO3��Ksp�ֱ�Ϊ1.0��10-33��1.9��10-13��2.2��10-11��
(l)����ԭ�����ʱ��������ΪCO2��������Ӧ�Ļ�ѧ����ʽΪ____��
(2)����ԭ�����ʵ���У��̵Ľ����ʽ����ͼ��ʾ����ͼ��֪�������õ����ʵ������Ϊ ____ ��

(3)��Һl�е���������ɼ��±���
��� | Mn2+ | Fe2+ | Fe3+ | Al3+ |
Ũ��/(mol��L-1) | 0.85 | 2.4��10-3 | 1.0��10-2 | 2.0��10-3 |
�����ӡ�ʱ�ȼ�MnO2��MnO2������Ϊ ___��
�ټӰ�ˮ����pHʹ�������ӳ�����ȫ��ͨ������˵��Al3+������ȫʱMn2+�Ƿ�ʼ���� ___��
(4)�����̡�ʱ������Ӧ�����ӷ���ʽΪ ___��
(5)�����̡��Ĺؼ�������2�㣺�ٽ�NH4HCO3��Һ����MnSO4��Һ�У��ڷ�Ӧ�յ�pH=7������ߵ��Լ��μ�˳��MnSO4��Һ����NH4HCO3����Һ�У���Ӧ�յ�pH>7�������ͬ���ĺ�����ú����____�����з�Ӧ�յ�pH<7�����ܵĺ����______��
(6)����Һ2�л��յõ��ĸ���Ʒ����;Ϊ________��