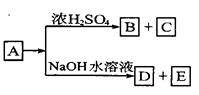
��Ŀ����
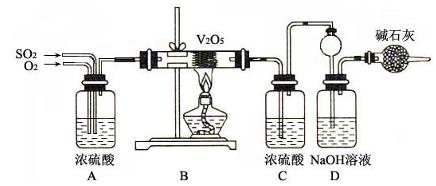
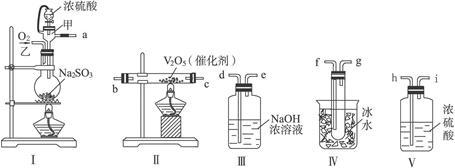
ij��ѧ����С����Ũ���ᡢMnO2������Cl2������Cl2��Ca(OH)2��Ӧ������Ư�ۣ�����֪��Ӧ2Cl2��2Ca(OH)2=Ca(ClO)2��CaCl2��2H2O������ӦΪ���ȷ�Ӧ���¶��Ը���������Ӧ6Cl2��
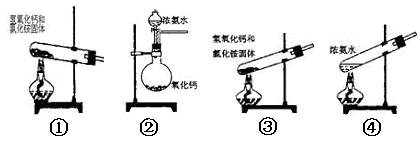
6Ca(OH)2=5CaCl2��Ca(ClO3)2��6H2O���ס��ҡ������˷ֱ��������ʵ��װ�ã�����ͼ��ʾ��

��1����ָ������װ�ø�����ȱ��������û�пɲ��
�ף�_______________________________________________________��
�ң�_______________________________________________________��
����_______________________________________________________��
��2��ͼ����A��B��������ɣ�����C��D��E��������ɣ�����F��G��ɣ��������ס��ҡ�������װ����ѡ�������IJ��֣������������ҵķ�����װһ�����Ƶ�ʵ��װ�ã�����ѡ���ֵı�ţ� ___________________________________��
��3��ʵ��������12mol��L��1��Ũ����100mL��������MnO2��Ӧ����������Ca��ClO��2�����ʵ�������С��0.15mol������ܵ���Ҫԭ���ǣ��ٶ�������Ӧ����Ӧ�����������Ӧ������ _______________________________________________________��
6Ca(OH)2=5CaCl2��Ca(ClO3)2��6H2O���ס��ҡ������˷ֱ��������ʵ��װ�ã�����ͼ��ʾ��

��1����ָ������װ�ø�����ȱ��������û�пɲ��
�ף�_______________________________________________________��
�ң�_______________________________________________________��
����_______________________________________________________��
��2��ͼ����A��B��������ɣ�����C��D��E��������ɣ�����F��G��ɣ��������ס��ҡ�������װ����ѡ�������IJ��֣������������ҵķ�����װһ�����Ƶ�ʵ��װ�ã�����ѡ���ֵı�ţ� ___________________________________��
��3��ʵ��������12mol��L��1��Ũ����100mL��������MnO2��Ӧ����������Ca��ClO��2�����ʵ�������С��0.15mol������ܵ���Ҫԭ���ǣ��ٶ�������Ӧ����Ӧ�����������Ӧ������ _______________________________________________________��
��1���ף��ٷ���װ�ô���װ�ò����ṩ�Ⱥ���������ҷ�Ӧ���ʲ�����ƣ���û��β������װ�ã�����Ⱦ������
�ң���û�н���װ�ã���ʹ�¶����߷�������Ӧ�����²��ﲻ������Ӧ����U�ιܣ���װ��ʯ�ҡ�
������û�н���װ�ã���ʹ�¶����߷�������Ӧ�����²��ﲻ������û��β������װ�ã�����Ⱦ������
��2��C��F����B��E
��3��MnO2ֻ������ŨHCl�����ŷ�Ӧ�Ľ��У�c��Cl������С����ԭ�Լ�������Ӧ���ܼ������С�
�ң���û�н���װ�ã���ʹ�¶����߷�������Ӧ�����²��ﲻ������Ӧ����U�ιܣ���װ��ʯ�ҡ�
������û�н���װ�ã���ʹ�¶����߷�������Ӧ�����²��ﲻ������û��β������װ�ã�����Ⱦ������
��2��C��F����B��E
��3��MnO2ֻ������ŨHCl�����ŷ�Ӧ�Ľ��У�c��Cl������С����ԭ�Լ�������Ӧ���ܼ������С�
����Ϊ�Ʊ�ʵ�鷽�������������ƣ�Ŀ�����ΪƯ�ۣ��������Ϣ֪��Ӧ�����ڵ��������½�����ԭ����ΪCl2�����Ʊ�װ�ñ������ṩ�Ⱥ���������ұ�����β������װ�ã��ݴ˿����ۼס��ҡ�������ʵ��װ�õ���ȱ�㡣ȡ����װ�õ��ŵ㼴Ϊ�Ʊ�Ư�۵����װ�á�

��ϰ��ϵ�д�
�����Ŀ