��Ŀ����
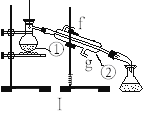
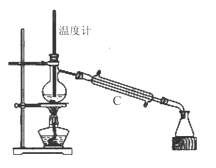
����Ŀ����������(NOCl,�۵�:-64.5 ��,�е�:-5.5 ��)��һ�ֻ�ɫ����,��ˮ��ˮ�⡣�����ںϳ���������ý�����м���ȡ�ʵ���ҿ���������һ�������ڳ��³�ѹ�ºϳɡ��Ʊ�װ������ͼ��ʾ:�����Т������ж�ΪŨ���ᣩ
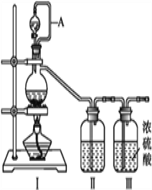

��1����ͬһװ����������III�ֱ��Ʊ����������NO��Cl2���±���ȱ�ٵ�ҩƷ��:
װ���� | װ���� | ||
��ƿ�� | ��Һ©���� | ||
�Ʊ�����Cl2 | MnO2 | Ũ���� | ��___ |
�Ʊ�����NO | Cu | ��___ | ˮ |
��2��A������____________________________��
��3��NOCl�ĵ���ʽ_________��
��4�������Ƶõ�NO��Cl2�Ʊ�NOCl,����ʽ��2NO + Cl2 = 2NOCl;װ������ͼ��ʾ:
��װ������˳��Ϊa��________(�������������ҷ���,��Сд��ĸ��ʾ)��
��װ�����������ɽ�һ������NO��Cl2��,��һ��������____________��
��װ����������β��ʱ��Cl2������Ӧ�����ӷ���ʽΪ________________��
��5������ͬѧ��������,�����ˮ��Ũ������Ũ����Ļ�����һ�������¸û���������������Ⱥ��������÷�Ӧ�Ļ�ѧ����ʽΪ__________________��
���𰸡� ����ʳ��ˮ ϡ���� ƽ��ѹǿ�����������𰸾��ɣ� 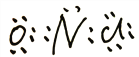 e��f(��f��e)��c��b��d ͨ���۲����ݵ������������ Cl2��2OH��===Cl����ClO����H2O HNO3(Ũ)+3HCl(Ũ)
e��f(��f��e)��c��b��d ͨ���۲����ݵ������������ Cl2��2OH��===Cl����ClO����H2O HNO3(Ũ)+3HCl(Ũ)![]() NOCl��+Cl2��+2H2O
NOCl��+Cl2��+2H2O
����������1���Ʊ�����Cl2�������ñ���ʳ��ˮ��ȥ�ӷ���HCl������Ũ����������壬����ȱ�ٱ���ʳ��ˮ���Ʊ�����NO�������ϡ���
��2��A����������ͨ��ƿ�ͷ�Һ©����ƽ��ѹǿ��ʹ����©����Һ��˳��������
��3��NOCl�и�ԭ���������8�����ȶ��ṹ����Nԭ����Oԭ�ӹ���2�Ե��ӣ���Clԭ�ӹ���1�Ե��ӣ������ʽΪ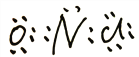
��4������������e�����������ռ��������ȣ���f����c���������ȣ�NOCl������ˮ��Ӧ����ˮ�Ȼ���������ˮ���������Է�ֹβ������װ���е�ˮ��������U�ܣ���b����d���ռ�β����
��ͨ���۲��������жϷ�Ӧ���ʣ��Ӷ�������������٣�
��Cl2����ˮ��������������ᣬ�Ӷ����������Ʒ�Ӧ��Cl2��2OH��===Cl����ClO����H2O
��5��HNO3(Ũ)��3HCl(Ũ)��Ӧ�У�NԪ�ر���ԭ��ClԪ�ر�����������NOCl�����Cl2�����Լ�H2O����ƽ����ʽ���˼��ɡ�

����Ŀ���������⡿����ȩ������ˮ���������л��ܼ����ܶ�Լ����ˮ���ܶȣ��ڼ��������·����绯��Ӧ�����Ʊ�����ȩ����ˮ���ܽ�Ȳ����������л��ܼ����ܶ�Լ����ˮ���ܶȣ��������ᡣ��Ӧԭ�����£�
2C6H5CHO+NaOH![]() C6H5CH2OH+C6H5COONa
C6H5CH2OH+C6H5COONa
C6H5COONa+HCl![]() C6H5COOH+NaCl
C6H5COOH+NaCl
������������������±���
����ȩ | ���״� | ������ | �� | |
�е�/�� | 178 | 205 | 249 | 80 |
�۵�/�� | 26 | -15 | 122 | 5.5 |
��������ˮ�е��ܽ�� | ||
17�� | 25�� | 100�� |
0.21g | 0.34g | 5.9g |
ʵ���������£�

��1���ڢ�����������1Сʱ����ͼ1�������м��Ȼ�Ϲ̶�װ��Ϊ������
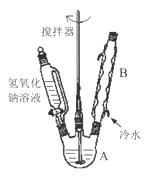
����A������Ϊ_______����������B��Ϊ����C��Ч������B��˵��ԭ��_______��
��2���������йط�Һ©����ʹ�ò���ȷ����_______
A.��Һ©����ʹ��֮ǰ��������Ƿ�©ˮ
B.��Һ©���ڵ�Һ�岻�ܹ��࣬����������
C.�����Һ©����������̨�Ͼ��ã��ֲ���������������з�Һ
D.��Һʱ���²�Һ�����������ر������������ձ��ٴ�����ʹ�ϲ�Һ������
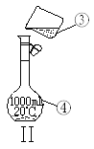
��3�����������÷�ˮԡ���������ٽ��в����ܣ���ͼ2�����ռ�______�����֡�ͼ2����һ�����Դ�����ȷ��Ӧ��Ϊ_____________��
��4������ʱ����ͼ3���ձ��б����ᾧ��ת�벼��©��ʱ�������ϻ�ճ���������壬��_____��ϴ�����ϲ����ľ��塣������ɺ���������ˮ�Ծ������ϴ�ӣ�ϴ��Ӧ____________��
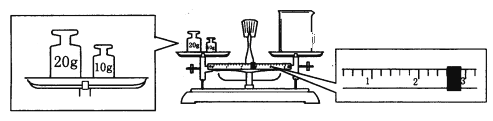
��5���õ�����ƽȷ��ȡ0.2440g����������ƿ�м�100mL����ˮ�ܽ⣨��Ҫʱ���Լ��ȣ�������0.1000mol/L�ı�����������Һ�ζ��������ı�����������Һ19.20mL��������Ĵ���Ϊ_____%��