��Ŀ����
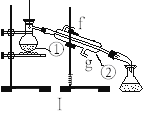
����Ŀ����ͼΪ����ʵ��װ�á�
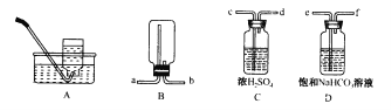
��д���������������ƣ� ��________________����________________ ��
�������١����У�ʹ��ʱ�������Ƿ�©ˮ����_____________��������ţ�
��������װ��I��ȡ����ˮ����ȱ�ٵ�������_____________���������������������ʵ�飬��ȴˮ��_______�ڽ���
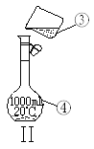
����������1.0 mol��L-1��NaOH��Һ240mL������װ��II��ijͬѧ���ƴ���Һʱת�Ʋ�����ʾ��ͼ��ͼ������������ֱ��� ___________________ ��_____________________ ��
��������Һʱ��һ����Է�Ϊ���¼������裺
�ٳ��� �ڼ��� ���ܽ� �ܵ�תҡ�� ��ת�� ��ϴ�� �߶��� ����ȴ
����ȷ�IJ���˳��Ϊ_____________________��
��ijͬѧ������һ������NaOH���壬������������ƽ�����ձ�����������ƽƽ����״̬��ͼ���ձ���ʵ������Ϊ______g����ͬѧӦ����______g NaOH��
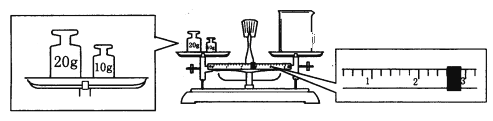
�������ƹ����У������������������ȷ�ģ����в���������Ũ��ƫ�ߵ���__________��
��û��ϴ���ձ��Ͳ����� ��ת����Һʱ������������Һ��������ƿ���� ������ƿ�����������������ˮ �ܶ���ʱ���ӿ̶��� �ݶ���ҡ�Ⱥ�����Һ������ڿ̶��ߣ��ٲ�����������ˮ���̶���
���𰸡�������ƿ�����ܢܾƾ���gδ�ò���������δ����250ml����ƿ�ڢ٢ۢ�ݢޢߢ� 27.410.0��
��������
��1������װ���е���Ҫ������������ƿ�������ܡ�ţ�ǹܡ���ƿ���ƾ��ƣ���Ϊ������ƿ��Ϊ�����ܣ��ʴ�Ϊ��������ƿ�������ܣ���2������ƿ��ʹ��ǰһ��Ҫ��©���ʴ�Ϊ��������3����ȡ����ˮ��ʵ����������̣������þƾ��ƣ��������¿��ǽ�ˮ�ڣ��Ͽ��dz�ˮ�ڣ��ʴ�Ϊ���ƾ��ƣ�g����4������һ�����ʵ���Ũ�ȵ���Һ�DZ����ò�������������ֹҺ���⽦�����ƶ���������Һ��ѡ�����������ƿ���ʴ�Ϊ��δ�ò�����������δ����250ml����ƿ����5�����Ʋ����м��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ������ʴ�Ϊ���ڢ٢ۢ�ݢޢߢ�����6������ƽ�ij���ԭ�����������������=�������������+����Ķ����������ձ���ʵ������Ϊ27.4g����������Һ�����Ϊ240ml��������ƿ�Ĺ��û��240ml��ֻ��ѡ��250ml��NaOH������m=cvM=1.0molL-1��0.25L��40g/mol=10.0g���ʴ�Ϊ��27.4��10.0����7����û��ϴ���ձ��Ͳ����������ʵ��������٣�Ũ��ƫС���ʢٴ���ת����Һʱ������������������ƿ���棬���ʵ��������٣�Ũ��ƫС���ʢڴ�������ƿ�����������������ˮ����Һ��������䣬Ũ�Ȳ��䣬�ʢ۴��ܶ���ʱ���ӿ̶��ߣ���Һ�����ƫС��Ũ��ƫ�ߣ��ʢ���ȷ���ݶ��ݺ�����ƿ������ҡ�ȣ����ú�Һ����ڿ̶��ߣ��ټ�ˮ���̶��ߣ���Һ�����ƫ��Ũ��ƫС���ʢݴ���Ϊ������

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ���÷�Ni--MH����������Ͽ��Ʊ����Ӽ����������壬�乤����������ͼ��ʾ:
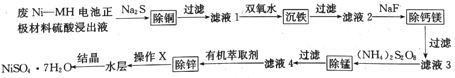
�ش���������:
(1)��Ni-NH������������������Һ�еijɷ�Ũ�����±���ʾ:
�ɷ� | Ni2+ | Fe2+ | Cu2+ | Ca2+ | Mg2+ | Mn2+ | H+ | SO42- |
Ũ��(mol/L) | c | 0.013 | 0.0007 | 0.0013 | 0.0030 | 0.004 | 0.01 | 1.8 |
��Һ��c(Ni2+)=_____mol/L(���������λ��Ч����)��
(2)�ڡ���Һ1���м���˫��ˮ�ɽ�Fe2+ת��Ϊ���ܵ�������(FeOOH),д����Ӧ�����ӷ���ʽ:___________��
(3)����Һ2���м���NaF�ɽ���Һ��Ca2+��Mg2+ת��Ϊ���ܵ�CaF2��MgF2�����������NaF�����á���Һ3����c(Mg2+):c(Ca2+)=0.67����MgF2���ܶȻ�Ϊ______[��֪Ksp(CaF2)=1.10��10-10]��
(4)�����̡�ʱ��(NH4)2S2O8�롰��Һ3���е�MnSO4��Ӧ���ɺ��̻�����R������識����ᣬ���������뻹ԭ�����ʵ���֮��Ϊ1:1����R�Ļ�ѧʽΪ_____��(����ĸ)
a.MnO2 b.Mn2O3 c.(NH4)2MnO4 d.Mn(OH)3
(5)����п��ʱ��Zn2+���л���ȡ��(��HA��ʾ)�γ���������ȡ���������ZnA2��2HA��
����д����ȡʱ��Ӧ�����ӷ���ʽ______________��
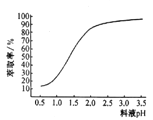
�ڡ���п��ʱ��п����ȡ������ҺpH�仯����ͼ��ʾ���Է���pH����ʱ��п����ȡ�����������ԭ����_____________________��
�ۡ�����X����������________________��
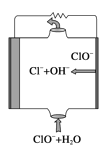
����Ŀ����������(NOCl,�۵�:-64.5 ��,�е�:-5.5 ��)��һ�ֻ�ɫ����,��ˮ��ˮ�⡣�����ںϳ���������ý�����м���ȡ�ʵ���ҿ���������һ�������ڳ��³�ѹ�ºϳɡ��Ʊ�װ������ͼ��ʾ:�����Т������ж�ΪŨ���ᣩ
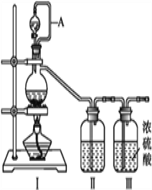

��1����ͬһװ����������III�ֱ��Ʊ����������NO��Cl2���±���ȱ�ٵ�ҩƷ��:
װ���� | װ���� | ||
��ƿ�� | ��Һ©���� | ||
�Ʊ�����Cl2 | MnO2 | Ũ���� | ��___ |
�Ʊ�����NO | Cu | ��___ | ˮ |
��2��A������____________________________��
��3��NOCl�ĵ���ʽ_________��
��4�������Ƶõ�NO��Cl2�Ʊ�NOCl,����ʽ��2NO + Cl2 = 2NOCl;װ������ͼ��ʾ:
��װ������˳��Ϊa��________(�������������ҷ���,��Сд��ĸ��ʾ)��
��װ�����������ɽ�һ������NO��Cl2��,��һ��������____________��
��װ����������β��ʱ��Cl2������Ӧ�����ӷ���ʽΪ________________��
��5������ͬѧ��������,�����ˮ��Ũ������Ũ����Ļ�����һ�������¸û���������������Ⱥ��������÷�Ӧ�Ļ�ѧ����ʽΪ__________________��