��Ŀ����
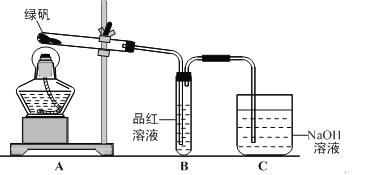
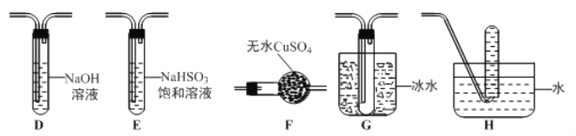
����Ŀ��ʵ���ǽ��л�ѧ�о�����Ҫ���ڣ������йػ�ѧʵ���˵��������ǣ� ��
A. �þƾ���������������������ۻ��������䣬˵��Al2O3���۵��Al���۵��
B. ��¯ˮ��CaSO4����Na2CO3��Һ���ݣ��������ܽ�ȥ����˵��Ksp��CaCO3>CaSO4
C. ������KMnO4��Һ��֤����Ļ�ԭ��
D. �����KSCN��FeCl3��Һ�м���NaOH��Һ���о���Ӧ��Ũ�ȶԻ�ѧƽ���Ӱ��
���𰸡�B
��������A����������������������Ӧ�������������ۻ��������䣬˵�����������۵���������ʣ���A˵����ȷ��B��CaSO4����ˮ��CaCO3������ˮ����̼������Һ���ݣ�����������ת������CaSO4��Na2CO3=CaCO3��Na2SO4����Ӧ���Ÿ����ܵķ�����У���Ksp(CaCO3)<Ksp(CaSO4)����B˵������C�����Ը��������Һ����ǿ�����ԣ�������ᣬ������������Һ��ɫ���������壬˵������Ļ�ԭ�ԣ���C˵����ȷ��D������Fe3����3SCN��![]() Fe(SCN)3�������������ƣ���Һ����ɫ��dz��˵�����ڴ�ƽ�⣬��D˵����ȷ��
Fe(SCN)3�������������ƣ���Һ����ɫ��dz��˵�����ڴ�ƽ�⣬��D˵����ȷ��

 ���100��1�ž�ϵ�д�
���100��1�ž�ϵ�д�����Ŀ���±���A��B��C��D���ֳ����л���������Ϣ��
���A | ���B | ���C | ���D |
�ٿ����ڹ�ʵ���� �ڱ���ģ��Ϊ
| ����C��H����Ԫ����� �����ģ��Ϊ
| �������г�����Һ̬�л��������̼ԭ�������л���A��ͬ ������Na��Ӧ����������NaOH��Ӧ | ����Է����������л���C��14 �������л���C�������� |
���ݱ�����Ϣ�ش���������:
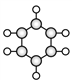
��1���л���A�ķ���ʽΪ_____________��
��2�������й��л���A��B��˵����ȷ����_______________
a��A��B����ʹ����KMnO4��Һ��ɫ
b��A��B���������е�ԭ����ͬһƽ����
c����������A��B��ȫȼ�գ���������������ͬ
d��A��B���Ӿ����й�����̼̼˫�����ܷ����ӳɷ�Ӧ
��3��д���л���C��ͬ���칹��Ľṹ��ʽ_________________��
��4����һ�������£��л���C���л���D��Ӧ�����ɾ���ˮ����ζ������E���仯ѧ��Ӧ����ʽΪ ______________________________________��ij��ʵ���У���5.0gDΪԭ�ϣ��Ƶ�4.4gE����D��ת����Ϊ______________��