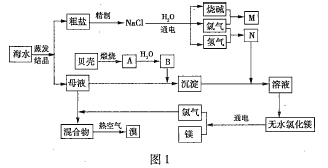
��Ŀ����
����Ŀ��50mL0.50mol/L������50mL0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȡ��ش��������⣺
��1����ʵ��װ���Ͽ���ͼ����ȱ��һ�ֲ���������___��
��2��ʵ���и���60mL0.50mol/L�����50mL0.55mol/LNaOH��Һ���з�Ӧ��������ʵ����ȣ������к���___(�������������ƫС������ƫ����)��
��3������ø÷�Ӧ���к���Ϊ57.3kJ/mol����д����ʾ�к��ȵ��Ȼ�ѧ����ʽ��___��
���𰸡����β�������� ��� H+(aq)+OH-(aq)=H2O(l) ��H=-57.3kJ/mol
��������
��1���������ȼƵĹ����֪��װ�õ�ȱ�������ǻ��β����������
��2����Ӧ�ų����������������Լ�������Ķ����йأ������к���ָ����ǿ���ǿ�Ӧ����1molˮʱ�ų����ȣ������������أ�
��3������ø÷�Ӧ���к���Ϊ57.3kJ/mol�����ʾ�к��ȵ��Ȼ�ѧ����ʽΪH+(aq)+OH-(aq)=H2O(l) ��H=-57.3kJ/mol��
��1���������ȼƵĹ����֪��װ�õ�ȱ�������ǻ��β�����������ʴ�Ϊ�����β����������
��2����Ӧ�ų����������������Լ�������Ķ����йأ�������60mL0.50mol/L�����50mL0.55mol/LNaOH��Һ���з�Ӧ��������ʵ����ȣ������������Ӧ����ˮ�����ʵ������ų�������ƫ�ߣ������к���ָ����ǿ���ǿ�Ӧ����1molˮʱ�ų����ȣ������������أ�������60mL0.50mol/L����50mL0.50mol/L�����������ʵ�飬����к�����ֵ��ȣ��ʴ�Ϊ����ȣ�
��3������ø÷�Ӧ���к���Ϊ57.3kJ/mol�����ʾ�к��ȵ��Ȼ�ѧ����ʽΪH+(aq)+OH-(aq)=H2O(l) ��H=-57.3kJ/mol���ʴ�Ϊ��H+(aq)+OH-(aq)=H2O(l) ��H=-57.3kJ/mol��

 �����Ծ���Ԫ���Ծ�ϵ�д�
�����Ծ���Ԫ���Ծ�ϵ�д�����Ŀ��Ϊ�˼���Ԫ�����ڱ�����150���꣬���Ϲ���2019�궨Ϊ�����ʻ�ѧԪ�����ڱ��������ش��������⣺
(1)Ag��Cu��ͬһ�壬��Ag�����ڱ���_____������s������p������d������ds��������[Ag(NH3)2]+��Ag+�յ�5s�����5p�����sp�ӻ��ɼ�����������ӵĿռ乹����_____��
(2)������Fe��Cu�IJ��ֵ��������ݣ������I2(Cu)����I2(Fe)����Ҫԭ��______��
Ԫ�� | Fe | Cu |
��һ������I1/kJ��mol��1 | 759 | 746 |
�ڶ�������I2/kJ��mol��1 | 1561 | 1958 |
(3)�����軯����ʳ���г��õĿ�������仯ѧʽΪK4[Fe(CN)6]��
��CN-�ĵ���ʽ��______��1mol���������к�������ĿΪ______��
�ڸ�������д��ڵ�������������______������ĸ����
A�������� B�����Ӽ� C�����ۼ� D����λ�� E����� F�����»���
(4)MnO���۵�(1660��)��MnS���۵�(1610��)�ߣ�����Ҫԭ����________��
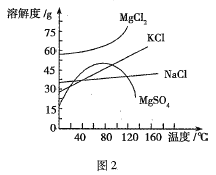
(5)������̫���ܵ�������л�����±����⻯Ǧ�װ�(CH3NH3PbI3)�뵼����Ϊ������ϣ�CH3NH3PbI3���и��ѿ�(AMX3)�������ṹ���侧����ͼ��ʾ��

��AMX3�����������������(M)���������±��������(X)�γ���������ṹ����M����_______λ�ã�X����______λ�ã���ѡ��������������������������������������������գ���
��CH3NH3PbI3����ľ�������Ϊa nm���侧���ܶ�Ϊdg��cm-3�����ӵ�������ֵNA�ļ������ʽΪ_________��
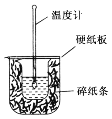
����Ŀ������������������۷���Ϣ�صijɷ�֮һ�������㽶����ζ��ʵ�����Ʊ������������ķ�Ӧ��װ��ʾ��ͼ���й��������£�
|
| ||||
��Է������� | �ܶ�/(g/cm-3) | �е�/�� | ˮ���ܽ��� | ||
���촼 | 88 | 0.8123 | 131 | �� | |
���� | 60 | 1.0492 | 118 | �� | |
���������� | 130 | 0.8670 | 142 | ���� | |
ʵ�鲽�裺��A�м���4.4g���촼��6.0g���ᡢ����Ũ�����2��3Ƭ���Ƭ����ʼ��������A������50min����ӦҺ�������º����Һ©���У��ֱ�������ˮ������̼��������Һ��ˮϴ�ӣ��ֳ��IJ������������ˮMgSO4���壬����Ƭ�̣����˳�ȥMgSO4���壬�����������ռ�140��143����֣�������������3.9g���ش��������⣺
��1������B��������__��
��2����ϴ�Ӳ����У���һ��ˮϴ����ҪĿ����__���ڶ���ˮϴ����ҪĿ����__��
��3����ϴ�ӡ���Һ�����У�Ӧ�����Ȼ���ã����ֲ��___�����ţ���
A.ֱ�ӽ������������ӷ�Һ©�����Ͽڵ���
B.ֱ�ӽ������������ӷ�Һ©�����¿ڷų�
C.�Ƚ�ˮ��ӷ�Һ©�����¿ڷų����ٽ��������������¿ڷų�
D.�Ƚ�ˮ��ӷ�Һ©�����¿ڷų����ٽ��������������Ͽڵ���
��4����ʵ���м�����������Ŀ����__��
��5������������У������Լ���ѡ��װ����ȷ����__�����ţ���
A.  B.
B.
C. D.
D.
��6����ʵ��IJ�����__��
��7���ڽ����������ʱ������130��㿪ʼ�ռ���֣���ʹʵ��IJ���ƫ__������������������������ԭ����__��