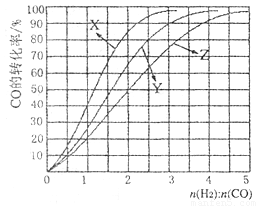
��Ŀ����
��1�������ʵ�����NO2��N2O���ڱ�״���µ����֮�� ������Oԭ�Ӹ���֮�� �����ߵ�����֮�� ��
��2��ͬ�¡�ͬѹ�£���������CH4��H2��O2��N2�������壬���������_ _ __�������� ������___ _���ܶ�������__ __��ԭ����������__ __�����ѧʽ��
������___ _���ܶ�������__ __��ԭ����������__ __�����ѧʽ��
��3��NaCl��MgCl2��AlCl3������Һ�������ʵ���Ũ����ͬ����������Һ�������ӵ����ʵ���Ũ��֮��Ϊ ���������ͬ�����ʵ���Ũ��֮��Ϊ1��2��3����������Һ�������ӵ����ʵ���Ũ��֮�� ���������ͬ�����ʵ���Ũ����ͬ��AgNO3��Һ�����Ũ������������Һǡ�÷�Ӧ��������������Һ�����֮��Ϊ ��
��10�֣���1��1��1 2��1 23��22 �� ��2�� H2��H2��O2�� H2
��3�� 1��2��3��1��4��9��6��3��2 ��ÿ��1�֣�
����

��ϰ��ϵ�д�
 �Ͻ�ƽСѧ��������ϵ�д�
�Ͻ�ƽСѧ��������ϵ�д� �Ƹ������������ϵ�д�
�Ƹ������������ϵ�д�
�����Ŀ
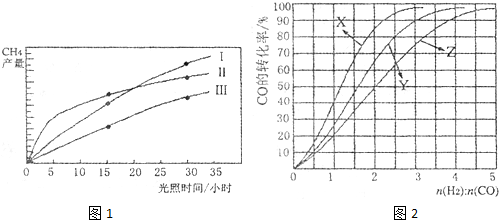

 ��
�� �ɴ�С��˳��Ϊ_________����Ӧ��ʼ���15Сʱ�ڣ��ڵ�_________�ִ����������£��ռ���CH4��ࡣ
�ɴ�С��˳��Ϊ_________����Ӧ��ʼ���15Сʱ�ڣ��ڵ�_________�ִ����������£��ռ���CH4��ࡣ CO(g) +3H2(g) ��H=+206kJ��mol-1���������ʵ�����CH4��H2O(g)����1L�����ܱ�������ij�¶��·�Ӧ�ﵽƽ�⣬��ʱ���CO�����ʵ���ΪO.10 mol,CH4��ƽ��ת����Ϊ91 %������¶��¸÷�Ӧ��ƽ�ⳣ��Ϊ_________ (������ȡ��������
CO(g) +3H2(g) ��H=+206kJ��mol-1���������ʵ�����CH4��H2O(g)����1L�����ܱ�������ij�¶��·�Ӧ�ﵽƽ�⣬��ʱ���CO�����ʵ���ΪO.10 mol,CH4��ƽ��ת����Ϊ91 %������¶��¸÷�Ӧ��ƽ�ⳣ��Ϊ_________ (������ȡ�������� �ֱ�Ϊ
�ֱ�Ϊ ��
�� ����CH3OH(l)����ȫȼ������CO(g)��H2O(l)���Ȼ�ѧ����ʽΪ_________��
����CH3OH(l)����ȫȼ������CO(g)��H2O(l)���Ȼ�ѧ����ʽΪ_________��

 ��
�� �ɴ�С��˳��Ϊ_________����Ӧ��ʼ���15Сʱ�ڣ��ڵ�_________�ִ����������£��ռ���CH4��ࡣ
�ɴ�С��˳��Ϊ_________����Ӧ��ʼ���15Сʱ�ڣ��ڵ�_________�ִ����������£��ռ���CH4��ࡣ CO(g)
+3H2(g) ��H=+206kJ��mol-1���������ʵ�����CH4��H2O(g)����1L�����ܱ�������ij�¶��·�Ӧ�ﵽƽ�⣬��ʱ���CO�����ʵ���ΪO.10 mol,CH4��ƽ��ת����Ϊ91 %������¶��¸÷�Ӧ��ƽ�ⳣ��Ϊ_________ (������ȡ��������
CO(g)
+3H2(g) ��H=+206kJ��mol-1���������ʵ�����CH4��H2O(g)����1L�����ܱ�������ij�¶��·�Ӧ�ﵽƽ�⣬��ʱ���CO�����ʵ���ΪO.10 mol,CH4��ƽ��ת����Ϊ91 %������¶��¸÷�Ӧ��ƽ�ⳣ��Ϊ_________ (������ȡ�������� �ֱ�Ϊ
�ֱ�Ϊ ��
�� ����CH3OH(l)����ȫȼ������CO(g)��H2O(l)���Ȼ�ѧ����ʽΪ_________��
����CH3OH(l)����ȫȼ������CO(g)��H2O(l)���Ȼ�ѧ����ʽΪ_________��