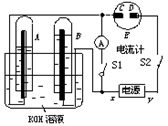
��Ŀ����
��ͼ��ʾ��ʵ��װ���У�EΪһ���õ��ۡ��⻯�غͷ�̪�����Һ��ʪ����ֽ��C��DΪ������ֽ���˵IJ��У�x��y�ֱ�Ϊֱ����Դ����������A��B�г���KOH��Һ������ʢ��KOH��Һ��ˮ���У��ٷֱ����һ��Ķ��Ե缫���жϵ�Դ����S�����պϿ���S����ֱͨ����һ��ʱ�������������ͼ��ʾ����ش��������⣺
(1)�����Դ������������xΪ�������� ��
(2)����ֽ��C�˸������۲쵽������������������ ���������������������������� �� ��
(3)д���缫��Ӧʽ��B�缫�������� ������������������ ������ ��
(4)�����һ��ʱ���A��B�о��������Χ�缫����ʱ�жϿ���S���պϿ���S����������Ƶ�ָ���Ƿ���ƫת���������� (�ƫת����ƫת��)��
(5)��������ָ��ƫת��д���йصĵ缫��Ӧ(��ָ�롰��ƫת�������ⲻ�ػش�)��
������������ ���������� ���� ���������������������� ���������� �������� ��
��������ָ�벻ƫת����˵������(��ָ�롰ƫת�������ⲻ�ػش�)������������ ��

�� �� ��1�֣� �� ��ֽ���� ��2�֣�
�� B����4OH�D�D4e=2H2O+O2�� ��2�֣�
�� ƫת ��1�֣� �� 2H2+4OH�D�D4e=4H2O ��2�֣� 2H2O+O2+4e=4OH�� ��2�֣�

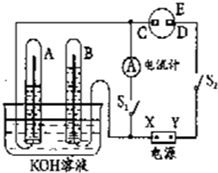 ����ͼ��ʾ��ʵ��װ���У�EΪһ���õ��ۡ��⻯�غͷ�̪�����Һ��ʪ����ֽ��C��DΪ������ֽ���˵IJ��У�X��Y�ֱ�Ϊֱ����Դ����������A��B�г���KOH��Һ������ʢ��KOH��Һ��ˮ���У��ٷֱ���벬�缫���жϵ�Դ����S1���պϿ���S2��ֱͨ����һ��ʱ�����ش��������⣺
����ͼ��ʾ��ʵ��װ���У�EΪһ���õ��ۡ��⻯�غͷ�̪�����Һ��ʪ����ֽ��C��DΪ������ֽ���˵IJ��У�X��Y�ֱ�Ϊֱ����Դ����������A��B�г���KOH��Һ������ʢ��KOH��Һ��ˮ���У��ٷֱ���벬�缫���жϵ�Դ����S1���պϿ���S2��ֱͨ����һ��ʱ�����ش��������⣺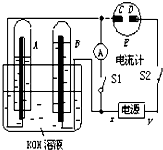 ��ͼ��ʾ��ʵ��װ���У�EΪһ���õ��ۡ��⻯�غͷ�̪�����Һ��ʪ����ֽ��C��DΪ������ֽ���˵IJ��У�x��y�ֱ�Ϊֱ����Դ����������A��B�г���KOH��Һ������ʢ��KOH��Һ��ˮ���У��ٷֱ����һ��Ķ��Ե缫���жϵ�Դ����S1���պϿ���S2��ֱͨ����һ��ʱ�������������ͼ��ʾ����ش��������⣺
��ͼ��ʾ��ʵ��װ���У�EΪһ���õ��ۡ��⻯�غͷ�̪�����Һ��ʪ����ֽ��C��DΪ������ֽ���˵IJ��У�x��y�ֱ�Ϊֱ����Դ����������A��B�г���KOH��Һ������ʢ��KOH��Һ��ˮ���У��ٷֱ����һ��Ķ��Ե缫���жϵ�Դ����S1���պϿ���S2��ֱͨ����һ��ʱ�������������ͼ��ʾ����ش��������⣺ ����ͼ��ʾ��ʵ��װ���У�A��ʢ��Ʒ����Һ��B ��ʢ��NaOH��Һ��
����ͼ��ʾ��ʵ��װ���У�A��ʢ��Ʒ����Һ��B ��ʢ��NaOH��Һ��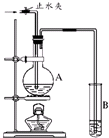 ����ͼ��ʾ��ʵ��װ���У�A��ʢ��Ʒ����Һ��B ��ʢ��NaOH��Һ��
����ͼ��ʾ��ʵ��װ���У�A��ʢ��Ʒ����Һ��B ��ʢ��NaOH��Һ��