��Ŀ����
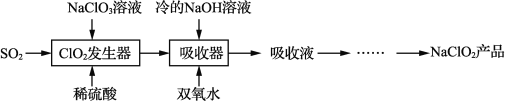
����Ŀ���绯ѧԭ���ڷ�ֹ������ʴ������ת���ȷ���Ӧ�ù㷺��
��1���ٸ����ں�ˮ���������绯ѧ��ʴ��������Ӧʽ��____________��
��ͼ1�У�Ϊ������բ�ŵĸ�ʴ������![]() ����ѡ��____________���
����ѡ��____________���![]() ����
����![]() ������
������
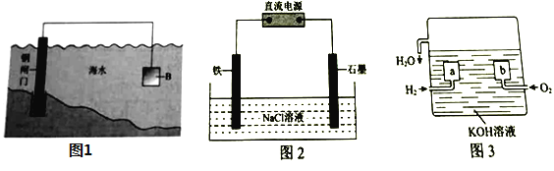
��2��ͼ2Ϊ��������ģ��ʵ��װ�ã�������_________��������������Ч���ķ����ǣ�ȡ�������缫��������Һ���Թ��У�___________����˵������Ч���á�
��3������ȼ�ϵ����һ�����͵Ļ�ѧ��Դ���乹����ͼ3��ʾ��![]() Ϊ���ʯī�缫��ͨ��������ɿ�϶���ݳ������ڵ缫����ŵ硣
Ϊ���ʯī�缫��ͨ��������ɿ�϶���ݳ������ڵ缫����ŵ硣
��![]() �ĵ缫��Ӧʽ��__________________��
�ĵ缫��Ӧʽ��__________________��
������ع�����![]() ˮ�����·��ͨ����___________
ˮ�����·��ͨ����___________![]() �ĵ��ӡ�
�ĵ��ӡ�
���𰸡�![]()
![]() �� �μ����軯�أ���
�� �μ����軯�أ���![]() ����Һ��������ɫ��������
����Һ��������ɫ�������� ![]() 40
40
��������
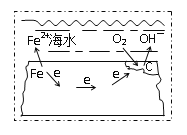
��1����ͼ���жϸ�װ�����õ���ԭ���ԭ������������������������������
��2����ͼ���жϸ�װ�����õ��ǵ���ԭ����������ӵ���������������������������Ч���ķ����Ǽ�����Һ���Ƿ�����������ӣ�
��3����д�缫��Ӧ����ʽע����Ի�����Ҫ����H+�����㷽���ҳ����Ӻ�ˮ�����ʵ�����ϵ���ɽ��
��1���ٸ���ʧȥ���ӷ���������Ӧ��ע��缫��Ӧ�У���ʧȥ����һ�����ɶ���������ӦʽΪ��![]() ��
��
��Ϊ�˼�����ˮ�Ը�բ��A�ĸ�ʴ������B�Ļ�����Ӧ��ǿ����������������������������������������������ѡZn��
��2����ӵ�������������������Ӧ�����Դ�ĸ�����������Ϊ��������Һ��û������������Ч���ã��������������ӵķ����������ǣ��μ����軯�أ���![]() ����Һ��������ɫ����������
����Һ��������ɫ����������
��3����a������ʧ������Ϊ��������ӦʽΪ��![]() ��
��
�����ܷ�Ӧ2H2+O2=2H2O�ɵã�2H2O~4e-������![]() ˮ�����·��ͨ����40
ˮ�����·��ͨ����40![]() �ĵ��ӡ�
�ĵ��ӡ�

 ������ѧ��ʱ��ҵϵ�д�
������ѧ��ʱ��ҵϵ�д� ���������ʱ��ѵϵ�д�
���������ʱ��ѵϵ�д� �㽭�¿γ���άĿ�������ʱ��ѵϵ�д�
�㽭�¿γ���άĿ�������ʱ��ѵϵ�д� ��������ϵ�д�
��������ϵ�д�����Ŀ��ijѧ����0.10 mol/L��NaOH��Һ�ζ�ijŨ�ȵ����ᡣ��¼�������£�
ʵ���� | NaOH��Һ��Ũ�ȣ�mol/L�� | ����NaOH��Һ�������mL�� | ����������Һ�������mL�� |
1 | 0.10 | 19.98 | 20.00 |
2 | 0.10 | 20.02 | 20.00 |
3 | 0.10 | 20.00 | 20.00 |
��1���ζ�ʱ���õ�ָʾ����__________________��
A��Ʒ����Һ B����̪��Һ C��ʯ����Һ
��2����ȥ��ʽ�ζ��������ݵķ���Ӧ���ò���_________��Ȼ�����ἷѹ������ʹ���첿�ֳ�����Һ��

��3���ζ�����ʱ���۾�Ӧע��____________________________________________��
��4���ζ��ﵽ�յ������____________________________________________________��
��5����ʽ�ζ��ܼ��첿�������ݣ��ζ�����ʧ���Բⶨ�����Ӱ����_______���ƫ�ߡ���ƫ�͡���Ӱ�족����ͬ������ʽ�ζ��ܵζ�ǰ���ӵζ����ӣ��Բⶨ�����Ӱ����_______����ƿ��ˮϴ����û������ϴ�������________��
��6�������������ݣ�������������Ũ��ԼΪ__________________��������λ��Ч���֣���
����Ŀ����֪��ѧ��Ӧ��:Fe(s)+CO2(g)![]() FeO(s)+CO(g),�仯ѧƽ�ⳣ��ΪK1;��ѧ��Ӧ��:Fe(s)+H2O(g)
FeO(s)+CO(g),�仯ѧƽ�ⳣ��ΪK1;��ѧ��Ӧ��:Fe(s)+H2O(g)![]() FeO(s)+H2(g),�仯ѧƽ�ⳣ��ΪK2,���¶�973 K��1173 K�������,K1��K2��ֵ�ֱ�����:
FeO(s)+H2(g),�仯ѧƽ�ⳣ��ΪK2,���¶�973 K��1173 K�������,K1��K2��ֵ�ֱ�����:
�¶� | K1 | K2 |
973 K | 1.47 | 2.38 |
1 173 K | 2.15 | 1.67 |
(1)ͨ�������е���ֵ�����ƶ�:��Ӧ����_______(��������������������)��Ӧ��
(2)���з�Ӧ��:CO2(g)+H2(g)![]() CO(g)+H2O(g),����д���÷�Ӧ��ƽ�ⳣ��K3�ı���ʽ:K3=______��
CO(g)+H2O(g),����д���÷�Ӧ��ƽ�ⳣ��K3�ı���ʽ:K3=______��
(3)���ݷ�Ӧ����ڿ��Ƶ���K1��K2��K3֮��Ĺ�ϵʽΪ__________,�ݴ˹�ϵʽ���ϱ�����,���ƶϳ���Ӧ����________(��������������������)��Ӧ��
(4)Ҫʹ��Ӧ����һ�������½�����ƽ��������Ӧ�����ƶ�,�ɲ�ȡ�Ĵ�ʩ��______ ��_____ (��д��ĸ���)��
A.��С��Ӧ�������ݻ� B.����Ӧ�������ݻ�
C.�����¶� D.ʹ�ú��ʵĴ���
E.�跨��Сƽ����ϵ�е�CO��Ũ��
(5)ͼ�ס��ҷֱ��ʾ��Ӧ����t1ʱ�̴ﵽƽ��,��t2ʱ����ı�ij�������������仯�����:

��ͼ����t2ʱ�̷����ı��������__________��
��ͼ����t2ʱ�̷����ı��������__________��