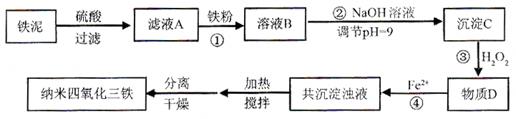
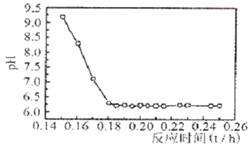
��Ŀ����
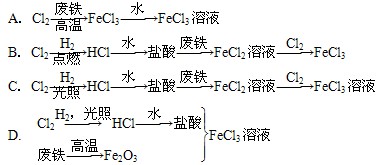
A��B��C��DΪ������ѧ��ѧ�г����ĵ��ʡ�����ʱAΪ����ɫ���壬BҲ�ǹ�����B�ɱ��Ż���C��D��˫ԭ�ӷ��ӵ����壬C�ʻ���ɫ��E��F��G��H��IΪ���ֻ����E������ˮ��FΪ���壬�Ҽ�������ˮ����ɫ������Һ��H����ˮ��û�ɫ���ػ�ɫ��Һ������֮�������·�Ӧ��ת����ϵ��
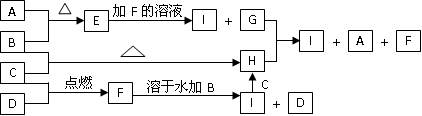
��1���������ʵĻ�ѧʽ��B________��C_______��E________��F_______��
��2������I��Һ����ɫ��_______ɫ��
��3����ʵ�鷽������H��I����__________�Լ���������_________________��
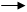 ��4��д��G+H I+A+F�����ӷ���ʽ_______________________________��
��4��д��G+H I+A+F�����ӷ���ʽ_______________________________��

��1���������ʵĻ�ѧʽ��B________��C_______��E________��F_______��
��2������I��Һ����ɫ��_______ɫ��
��3����ʵ�鷽������H��I����__________�Լ���������_________________��
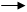 ��4��д��G+H I+A+F�����ӷ���ʽ_______________________________��
��4��д��G+H I+A+F�����ӷ���ʽ_______________________________����1��B��Fe C��Cl2 E��FeS F��HCl
��2��dz��ɫ
��3��KSCN ��Һ��Ѫ��ɫ
��4��2Fe3+ + H2S = 2Fe2+ + S + 2H+
��2��dz��ɫ
��3��KSCN ��Һ��Ѫ��ɫ
��4��2Fe3+ + H2S = 2Fe2+ + S + 2H+
AΪ����ɫ���壬AΪS; BҲ�ǹ�����B�ɱ��Ż�, BΪFe; C�ʻ���ɫ CΪCl2�� DΪH2�� EΪFeS; FΪHCl; GΪH2S; HΪFeCl3 ;IΪFeCl2
��1��B��Fe C��Cl2 E��FeS F��HCl
��2��dz��ɫ
��3��KSCN ��Һ��Ѫ��ɫ
��4��2Fe3+ + H2S = 2Fe2+ + S + 2H+

��ϰ��ϵ�д�
 ��ҵ����ϵ�д�
��ҵ����ϵ�д� ͬ��ѧ��һ�ζ���ϵ�д�
ͬ��ѧ��һ�ζ���ϵ�д� �����ܾ�ϵ�д�
�����ܾ�ϵ�д�
�����Ŀ