��Ŀ����
(12��)
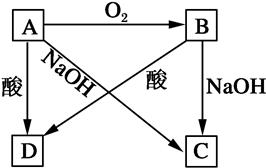
������Ԫ����ɵĵ���X2��Y����״����X2���ܶ�Ϊ3.17g��L��1�������£�YΪdz��ɫ���塣Z��һ�ֻ������ɫ��Ӧ��dz��ɫ(���ܲ���)��0.1mol��L��1 Z��ˮ��ҺpH=13��X2��Y ��Z֮��������ת����ϵ(��������������ȥ)
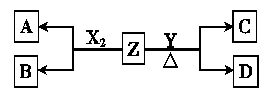
 ��1��д�������µ���X2��Z��Ӧ�����ӷ���ʽ
��1��д�������µ���X2��Z��Ӧ�����ӷ���ʽ
��2����֪C�������ᷴӦ������ʹƷ����Һ��ɫ������
��D�Ļ�ѧʽ�� ��D��ˮ��ҺpH��7��ԭ����(�����ӷ���ʽ��ʾ)
�ڽ�20mL 0.5mol��L��1 C��Һ��μ��뵽20 mL 0.2mol��L��1 KMnO4��Һ(�����ữ)�У���Һǡ����Ϊ��ɫ��д����Ӧ�����ӷ���ʽ
���±���Ԫ�����ڱ���һ���֣��������е���ĸ�ֱ����ijһԪ�أ�
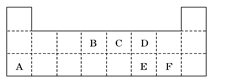
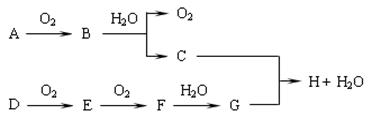
ij�ֽ���Ԫ�صĵ���G�����Է�������ͼ��ʾת����
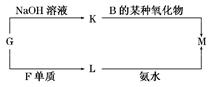
���л�����M��һ�ְ�ɫ��״������K����Һ�����B��ij�������ﷴӦ�Ļ�ѧ����ʽΪ___________________________��һ���������ǽ���������GԪ�غ�CԪ����ɣ��仯ѧʽΪ ��
������Ԫ����ɵĵ���X2��Y����״����X2���ܶ�Ϊ3.17g��L��1�������£�YΪdz��ɫ���塣Z��һ�ֻ������ɫ��Ӧ��dz��ɫ(���ܲ���)��0.1mol��L��1 Z��ˮ��ҺpH=13��X2��Y ��Z֮��������ת����ϵ(��������������ȥ)
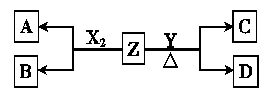
 ��1��д�������µ���X2��Z��Ӧ�����ӷ���ʽ
��1��д�������µ���X2��Z��Ӧ�����ӷ���ʽ ��2����֪C�������ᷴӦ������ʹƷ����Һ��ɫ������
��D�Ļ�ѧʽ�� ��D��ˮ��ҺpH��7��ԭ����(�����ӷ���ʽ��ʾ)
�ڽ�20mL 0.5mol��L��1 C��Һ��μ��뵽20 mL 0.2mol��L��1 KMnO4��Һ(�����ữ)�У���Һǡ����Ϊ��ɫ��д����Ӧ�����ӷ���ʽ
���±���Ԫ�����ڱ���һ���֣��������е���ĸ�ֱ����ijһԪ�أ�

ij�ֽ���Ԫ�صĵ���G�����Է�������ͼ��ʾת����

���л�����M��һ�ְ�ɫ��״������K����Һ�����B��ij�������ﷴӦ�Ļ�ѧ����ʽΪ___________________________��һ���������ǽ���������GԪ�غ�CԪ����ɣ��仯ѧʽΪ ��
��1�� Cl2 �� 2OH�C = Cl�C �� ClO�C �� H2O
��2�� �� K2S S2�C �� H2O HS�C �� OH�C
HS�C �� OH�C
�� 5SO32�C �� 2MnO4�C �� 6H+ = 5SO42 �C �� 2Mn2+ �� 3H2O
��NaAlO2 �� CO2 �� 2H2O = NaHCO3 �� Al(OH)3�� ��AlN
��2�� �� K2S S2�C �� H2O
 HS�C �� OH�C
HS�C �� OH�C�� 5SO32�C �� 2MnO4�C �� 6H+ = 5SO42 �C �� 2Mn2+ �� 3H2O
��NaAlO2 �� CO2 �� 2H2O = NaHCO3 �� Al(OH)3�� ��AlN
�����������M(X2) = 3.17g��L��1��22.4L��mol�C1 =" 71" g��mol�C1������XΪ��Ԫ�أ������£�������Ԫ��Y�ĵ���Ϊ����ɫ���壬��YΪ��Ԫ�أ�Z����ɫ��Ӧ��dz��ɫ��˵��Z�к��м�Ԫ�أ�0.1mol��L��1 Z��ˮ��ҺpH=13˵��ZΪǿ���ZΪKOH����X2��Y ��Z֮���ת����ϵͼ��֪X2��Z�ķ�ӦΪ��Cl2 + 2KOH =" KCl" + KClO + H2O��Y ��Z�ڼ��������µķ�ӦΪ��3S + 6KOH = K2S + 2K2SO3 + 3H2O�����������ɵã���1�� д�������µ���X2��Z��Ӧ�����ӷ���ʽ��Cl2 �� 2OH�C = Cl�C �� ClO�C �� H2O ��2��CΪK2SO3�������ᷴӦ����SO2ʹƷ����Һ��ɫ����DΪK2S����D�Ļ�ѧʽΪK2S����ˮ��ҺpH��7������ΪS2�Cˮ��ʼ��ԣ�ˮ������ӷ���ʽΪ��S2�C �� H2O
 HS�C �� OH�C����n(K2SO3)= 0.02L��0.5mol��L�C1 = 0.01mol��n(KMnO4) = 0.02L��0.2mol��L��1 = 0.004mol��n(K2SO3):n(KMnO4) =5:2������KMnO4����ǿ�����ԣ��ܰ�����ΪSO42�C����������ԭΪMnO4�C������ƽ������ӷ���ʽΪ��5SO32�C �� 2MnO4�C �� 6H+ = 5SO42 �C �� 2Mn2+ �� 3H2O��
HS�C �� OH�C����n(K2SO3)= 0.02L��0.5mol��L�C1 = 0.01mol��n(KMnO4) = 0.02L��0.2mol��L��1 = 0.004mol��n(K2SO3):n(KMnO4) =5:2������KMnO4����ǿ�����ԣ��ܰ�����ΪSO42�C����������ԭΪMnO4�C������ƽ������ӷ���ʽΪ��5SO32�C �� 2MnO4�C �� 6H+ = 5SO42 �C �� 2Mn2+ �� 3H2O����������G����NaOH��Һ��Ӧ����GΪ��Ԫ�أ���Ԫ�����ڱ�������Ԫ�ص�λ�ÿ�֪��BΪ̼Ԫ�أ�CΪ��Ԫ�أ�FΪ��Ԫ�أ��ִ��й����ʵ�ת����ϵͼ��֪��KΪƫ�����ƣ�LΪ�Ȼ�����KΪ������������NaAlO2��CO2��Ӧ�Ļ�ѧ����ʽΪ��NaAlO2 �� CO2 �� 2H2O = NaHCO3 �� Al(OH)3�������뵪Ԫ���γɻ�����ʱ������+3�ۣ���Ϊ�C3�ۣ��仯ѧʽΪAlN��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ

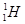 ��
�� ��
�� ��H+��H2��
��H+��H2��