题目内容
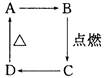
有A、B、C三种非金属元素,A原子与B原子的核外电子总数恰好等于C原子的一半,B原子与C原子的最外层电子数相同,它们各自的质子数与中子数均相等,1 mol C溶于苛性钠溶液中产生2 mol气体。
(1)它们的名称分别是:A_____________,B_____________,C_____________。
(2)写出1 mol C溶于苛性钠溶液中产生2 mol气体的化学反应方程式:_________________。
(3)B、C的最高价氧化物对应水化物中酸性较强的是_________________(写酸的化学式),在自然界中能稳定存在的A的同位素原子名称为__________________________。
(4)C的氧化物与B反应生成C单质的化学反应方程式为____________________________,C的氧化物与纯碱在一定条件下反应的化学方程式为________________________________。
(1)它们的名称分别是:A_____________,B_____________,C_____________。
(2)写出1 mol C溶于苛性钠溶液中产生2 mol气体的化学反应方程式:_________________。
(3)B、C的最高价氧化物对应水化物中酸性较强的是_________________(写酸的化学式),在自然界中能稳定存在的A的同位素原子名称为__________________________。
(4)C的氧化物与B反应生成C单质的化学反应方程式为____________________________,C的氧化物与纯碱在一定条件下反应的化学方程式为________________________________。
(1)氢 碳 硅
(2)Si+2NaOH+H2O====Na2SiO3+2H2↑
(3)H2CO3 氕
(4)SiO2+2C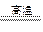 Si+2CO↑
Si+2CO↑
SiO2+Na2CO3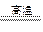 Na2SiO3+CO2↑
Na2SiO3+CO2↑
(2)Si+2NaOH+H2O====Na2SiO3+2H2↑
(3)H2CO3 氕
(4)SiO2+2C
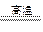 Si+2CO↑
Si+2CO↑SiO2+Na2CO3
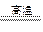 Na2SiO3+CO2↑
Na2SiO3+CO2↑A、B、C都是非金属元素,1 mol C溶于苛性钠溶液中产生2 mol气体,可推知C为硅。再结合A与B的核外电子总数恰好等于C的一半,B与C的最外层电子数相同,它们各自的质子数与中子数均相等,可推知A为氢,B为碳。

练习册系列答案
相关题目