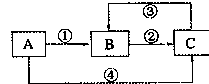
��Ŀ����
��13�֣�����A������ͼ��ʾת����ϵ��������Ϊ�������ʣ������£�����G��Ũ��Һ�з����ۻ��� F ����Һ��ֻ����һ�����ʣ��еķ�Ӧ������ˮ��Һ�н��У��еķ�Ӧ��������δȫ����������Ӧ����Ҳδע�������������������������ע����������¸���ĸ���������ʿ��ܲ�ͬ��
�ش��������⣺
��l����һ��������������������ֱ�պȡ A��G��Ũ��Һ��ʹ���ǽӽ�ʱ���д����������ɣ���Ϊ��ɫ��Ӧ�ʻ�ɫ�Ľ������ʣ� D �� F ����Һ���ʼ��ԡ��ٷ�Ӧ��Ļ�ѧ����ʽΪ_______________________ ____________��
��D���ҷ�Ӧ�����ӷ���ʽΪ___________________________ _________
��2���ڶ�������� �������ֵ���ֱ�ӻ��ϵõ���B Ϊ����ɫ���壻 CΪ��ɫ���壬���γ�����Ĵ�����Ⱦ��֮һ��D ��ˮ��Һ�����������ữ��AgNO3��Һ�а�ɫ�������ɡ���
�ٹ�ҵ�ϣ���Ӧ I�Ļ�ѧ����ʽΪ______________________________________
�����豸����Ϊ��
��D���ҷ�Ӧ�����ӷ���ʽΪ____________________________________
�� ���������D��Һ�������ӵķ��� ��
�� ��A����Է�������Ϊ120����Ӧ���Ϊ��ȫת������ȡm�˺�A���������ʵ���Ʒ�����������̳�ַ�Ӧ�����ʲ����뷴Ӧ�����õ��ܶ�Ϊ��g/cm3��������������Ϊa% ��G��Һn mL�������Ʒ��A������������ ���г�����ʽ���ɣ���
��13�֣���1����3NO2+H2O=NO+2HNO3 ��2�֣���2Al+2OH��+2H2O �� 2AlO2��+3H2����2�֣�
��2���� 4FeS2+11O2=2Fe2O3+8SO2����Ӧ���������£���2�֣� ����¯ ��1�֣�
��2Fe3+ + Fe ��3Fe2+ ��2�֣�
��ȡ����G��Һ��һ�Թ��У������е���1��2�����軯����Һ����Һ���ֺ�ɫ���������������ӣ�2�֣�
�� ���ޡ���100%��Ҳ���ԣ�����������Ҳ���֣� ��2�֣�
����:

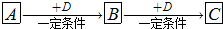
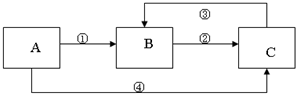
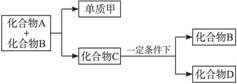
 �� 12�֣�A��B��C���������о�����ͬһ��Ԫ�أ�����֮��������ͼ��ʾ��ת����ϵ�����ַ�Ӧ��������ȥ����
�� 12�֣�A��B��C���������о�����ͬһ��Ԫ�أ�����֮��������ͼ��ʾ��ת����ϵ�����ַ�Ӧ��������ȥ����