��Ŀ����
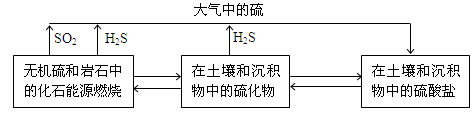
��14�֣���������Ȼ���еIJ���ѭ����ϵ���¡�
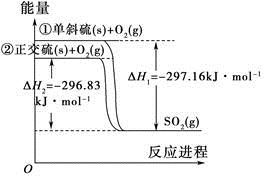
��1��H2S�ڿ����п���ȼ�ա�
��֪�� 2H2S(g) + O2(g) 2S(s) + 2H2O(g) ��H= ��442.38 kJ/mol ��
2S(s) + 2H2O(g) ��H= ��442.38 kJ/mol ��
S(s) + O2(g) SO2(g) ��H=��297.04 kJ/mol ��
SO2(g) ��H=��297.04 kJ/mol ��
H2S(g)��O2(g)��Ӧ����SO2(g)��H2O(g)���Ȼ�ѧ����ʽ�� ��
��2��SO2�Ǵ�����Ⱦ���ˮ�������õ�����SO2����������������¡�
�� SO2���ں�ˮ����H2SO3��H2SO3���ջ�����SO32��������뷽��ʽ�� ��
�� SO32�����Ա���ˮ�е��ܽ�������ΪSO42������ˮ��pH�� ������ߡ� �������䡱���͡�����
�� Ϊ������ˮ��pH���ɼ������ʵĺ�ˮ��ʹ���е�HCO3�����뷴Ӧ���䷴Ӧ�����ӷ���ʽ�� ��
�� ��������Ӧ��ͬʱ��Ҫ���������������ԭ���� ��
��3����Ȼ��ر���ԭ��ͭ�����ᆳ�������������ú���CuSO4��Һ���������������������ܵ�ZnS������ת��Ϊͭ����CuS�����û�ѧ�����ʾ��ZnSת��ΪCuS�Ĺ��̣� ��
��4��SO2��O2��H2SO4��Һ�п��Թ���ԭ��أ��为����Ӧʽ�� ��

��1��H2S�ڿ����п���ȼ�ա�
��֪�� 2H2S(g) + O2(g)
 2S(s) + 2H2O(g) ��H= ��442.38 kJ/mol ��
2S(s) + 2H2O(g) ��H= ��442.38 kJ/mol ��S(s) + O2(g)
 SO2(g) ��H=��297.04 kJ/mol ��
SO2(g) ��H=��297.04 kJ/mol ��H2S(g)��O2(g)��Ӧ����SO2(g)��H2O(g)���Ȼ�ѧ����ʽ�� ��
��2��SO2�Ǵ�����Ⱦ���ˮ�������õ�����SO2����������������¡�
�� SO2���ں�ˮ����H2SO3��H2SO3���ջ�����SO32��������뷽��ʽ�� ��
�� SO32�����Ա���ˮ�е��ܽ�������ΪSO42������ˮ��pH�� ������ߡ� �������䡱���͡�����
�� Ϊ������ˮ��pH���ɼ������ʵĺ�ˮ��ʹ���е�HCO3�����뷴Ӧ���䷴Ӧ�����ӷ���ʽ�� ��
�� ��������Ӧ��ͬʱ��Ҫ���������������ԭ���� ��
��3����Ȼ��ر���ԭ��ͭ�����ᆳ�������������ú���CuSO4��Һ���������������������ܵ�ZnS������ת��Ϊͭ����CuS�����û�ѧ�����ʾ��ZnSת��ΪCuS�Ĺ��̣� ��
��4��SO2��O2��H2SO4��Һ�п��Թ���ԭ��أ��为����Ӧʽ�� ��
��14�֣�ÿ��2�֣�
��1��2H2S(g)+3O2(g) 2SO2(g)+2H2O(g) ��H= ��1036.46 kJ/mol ������ʽ�ͼ����1�֣�
2SO2(g)+2H2O(g) ��H= ��1036.46 kJ/mol ������ʽ�ͼ����1�֣�
��2���� H2SO3 HSO3��+ H+ HSO3��
HSO3��+ H+ HSO3�� SO3 2��+ H+ ��ÿ��1�֣�
SO3 2��+ H+ ��ÿ��1�֣�
�� ����
�� HCO3 ��+ H+ CO2 ��+ H2O������1�֣���ƽ1�֣�
CO2 ��+ H2O������1�֣���ƽ1�֣�
���������ˮ���ܽ�������SO32-������ΪSO42-��������ƽ��H2SO3 HSO3��+ H+ HSO3��
HSO3��+ H+ HSO3�� SO3 2��+ H+ �����ƶ���1�֣�����߶��������ת���ʣ�ͬʱ�ӿ췴Ӧ���ʵ����ã�1�֣���
SO3 2��+ H+ �����ƶ���1�֣�����߶��������ת���ʣ�ͬʱ�ӿ췴Ӧ���ʵ����ã�1�֣���
��3��

��4��������SO2 - 2e��+ 2H2O SO4 2��+ 4H+������1�֣���ƽ1�֣�
SO4 2��+ 4H+������1�֣���ƽ1�֣�
��1��2H2S(g)+3O2(g)
 2SO2(g)+2H2O(g) ��H= ��1036.46 kJ/mol ������ʽ�ͼ����1�֣�
2SO2(g)+2H2O(g) ��H= ��1036.46 kJ/mol ������ʽ�ͼ����1�֣���2���� H2SO3
 HSO3��+ H+ HSO3��
HSO3��+ H+ HSO3�� SO3 2��+ H+ ��ÿ��1�֣�
SO3 2��+ H+ ��ÿ��1�֣��� ����
�� HCO3 ��+ H+
 CO2 ��+ H2O������1�֣���ƽ1�֣�
CO2 ��+ H2O������1�֣���ƽ1�֣����������ˮ���ܽ�������SO32-������ΪSO42-��������ƽ��H2SO3
 HSO3��+ H+ HSO3��
HSO3��+ H+ HSO3�� SO3 2��+ H+ �����ƶ���1�֣�����߶��������ת���ʣ�ͬʱ�ӿ췴Ӧ���ʵ����ã�1�֣���
SO3 2��+ H+ �����ƶ���1�֣�����߶��������ת���ʣ�ͬʱ�ӿ췴Ӧ���ʵ����ã�1�֣�����3��

��4��������SO2 - 2e��+ 2H2O
 SO4 2��+ 4H+������1�֣���ƽ1�֣�
SO4 2��+ 4H+������1�֣���ƽ1�֣������������1�����ݸ�˹���ɵ�Ŀ�귽��ʽ=��+2���ڣ�����H2S(g)��O2(g)��Ӧ����SO2(g)��H2O(g)���Ȼ�ѧ����ʽ��2H2S(g)+3O2(g)
 2SO2(g)+2H2O(g) ��H= ��1036.46 kJ/mol
2SO2(g)+2H2O(g) ��H= ��1036.46 kJ/mol ��2���������������ᣬ�ֲ������SO3 2�������뷽��ʽ��H2SO3
 HSO3��+ H+ HSO3��
HSO3��+ H+ HSO3�� SO3 2��+ H+
SO3 2��+ H+ ��SO32�����Ա���ˮ�е��ܽ�������ΪSO42����������ǿ�ᣬ��Һ�е�������Ũ������pH���ͣ�
�����ʵĺ�ˮ�����ԣ���HCO3����Ӧ���ɶ�����̼��ˮ�����ӷ���ʽΪHCO3 ��+ H+
 CO2 ��+ H2O
CO2 ��+ H2O�ܺ�ˮ�������õ�����SO2���������������������������ߺ�ˮ�еĺ���������SO32-������ΪSO42-��������ƽ��H2SO3
 HSO3��+ H+ HSO3��
HSO3��+ H+ HSO3�� SO3 2��+ H+ �����ƶ�����߶��������ת���ʣ�ͬʱ�ӿ췴Ӧ���ʵ����ã�
SO3 2��+ H+ �����ƶ�����߶��������ת���ʣ�ͬʱ�ӿ췴Ӧ���ʵ����ã���3��CuS��ZnS�����ܣ���п����ZnS(s)
 Zn2+(aq)+S2��(aq)ƽ�⣬����������ͭ��Һʱ��S2����Cu2+�������CuS������ʹ�ܽ�ƽ�������ƶ�������ZnSȫ��ת��ΪCuS���û�ѧ�����ʾΪ
Zn2+(aq)+S2��(aq)ƽ�⣬����������ͭ��Һʱ��S2����Cu2+�������CuS������ʹ�ܽ�ƽ�������ƶ�������ZnSȫ��ת��ΪCuS���û�ѧ�����ʾΪ
��4����������������Ӧ��Ԫ�ػ��ϼ����ߣ������Ƕ��������ڸ���������Ӧ��������������ӣ��缫��ӦʽΪSO2 - 2e��+ 2H2O
 SO4 2��+ 4H+
SO4 2��+ 4H+
��ϰ��ϵ�д�
�����Ŀ
 H��ͬ
H��ͬ 2NH3(g)
2NH3(g)