��Ŀ����
ȡ�����0.05mol/L��Ba(OH)2��Һ���ֱ�װ����Т٢ڢܱۢ�ŵ�4����ƿ�У����ټ�ˮϡ�͵�ԭ�����2�����ڢڢ��зֱ�ͨ��������CO2���������գ��ֱ��ڢ٢��еμӷ�̪��ָʾ�����ڢۢ��еμӼ�����ָʾ������HCl��Һ�ζ�����4����Һ��������HCl��Һ�������
[����]
A���٣��ڣ��ۣ���
B���ڣ��ۣ��٣���
C���٣��ڣ��ۣ���
D���ڣ��ۣ��٣���
�𰸣�D

��ϰ��ϵ�д�
�����Ŀ
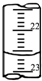 ������ʱ1L 0.01mol?L-1������������Һ��pHΪ
������ʱ1L 0.01mol?L-1������������Һ��pHΪ