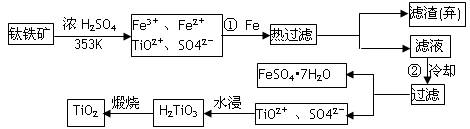
��Ŀ����
��1����֪��Fe(s)+
O2(g)=FeO(s)��H=-272.0kJ?mol-1
2Al(s)+
O2(g)=Al2O3(s)��H=-1675.7kJ?mol-1
Al��FeO�������ȷ�Ӧ���Ȼ�ѧ����ʽ��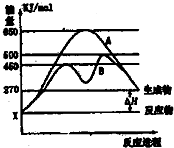
��2��ij���淴Ӧ�ڲ�ͬ�����µķ�Ӧ���̷ֱ�ΪA��B����ͼ��ʾ��
�پ�ͼ�жϸ÷�Ӧ��
������B���̱����˷�Ӧ���õ�����Ϊ
A�������¶�
B������Ӧ���Ũ��
C�������¶�
D��ʹ�ô���
��3��1000��ʱ���������������������з�Ӧ��
Na2SO4��S��+4H2��g���TNa2S��s��+4H2O��g��
�÷�Ӧ��ƽ�ⳣ������ʽΪ
��
��֪K1000����K1200������������ϵ�¶ȣ���������ƽ����Է���������
��4�������£����ȡ0.1mol?L-1 HA��Һ��0.1mol?L-1 NaOH��Һ�������ϣ���Ϻ���Һ����ı仯���Բ��ƣ�����û��Һ��pH=8��
�ٻ��Һ����ˮ�������OH-Ũ����0.1mol?L-1 NaOH��Һ����ˮ�������OH-Ũ��֮��Ϊ
����֪NH4A��ҺΪ���ԣ���֪��HA��Һ�ӵ�Na2CO3��Һ��������ų������ƶϣ�NH4��2CO3��Һ��pH
��ͬ�¶��£������ʵ���Ũ�ȵ�������������Һ��pH�ɴ�С������˳��Ϊ
a��NH4HCO3 b��NH4A c����NH4��2CO3 d��NH4Cl��
| 1 |
| 2 |
2Al(s)+
| 3 |
| 2 |
Al��FeO�������ȷ�Ӧ���Ȼ�ѧ����ʽ��
2Al��s��+3FeO��s���TAl2O3��s��+3Fe��s����H=-859.7 kJ?mol-1
2Al��s��+3FeO��s���TAl2O3��s��+3Fe��s����H=-859.7 kJ?mol-1
��
��2��ij���淴Ӧ�ڲ�ͬ�����µķ�Ӧ���̷ֱ�ΪA��B����ͼ��ʾ��
�پ�ͼ�жϸ÷�Ӧ��
ϩ
ϩ
��������š����ȷ�Ӧ������Ӧ�ﵽƽ��������������䣬�����¶ȣ���Ӧ���ת������С
��С
�����������С�����䡱����������B���̱����˷�Ӧ���õ�����Ϊ
D
D
����ѡ����ţ���A�������¶�
B������Ӧ���Ũ��
C�������¶�
D��ʹ�ô���
��3��1000��ʱ���������������������з�Ӧ��
Na2SO4��S��+4H2��g���TNa2S��s��+4H2O��g��
�÷�Ӧ��ƽ�ⳣ������ʽΪ
| c4(H2O) |
| c4(H2) |
| c4(H2O) |
| c4(H2) |
��֪K1000����K1200������������ϵ�¶ȣ���������ƽ����Է���������
����
����
�����������С�����䡱������4�������£����ȡ0.1mol?L-1 HA��Һ��0.1mol?L-1 NaOH��Һ�������ϣ���Ϻ���Һ����ı仯���Բ��ƣ�����û��Һ��pH=8��
�ٻ��Һ����ˮ�������OH-Ũ����0.1mol?L-1 NaOH��Һ����ˮ�������OH-Ũ��֮��Ϊ
107��1
107��1
������֪NH4A��ҺΪ���ԣ���֪��HA��Һ�ӵ�Na2CO3��Һ��������ų������ƶϣ�NH4��2CO3��Һ��pH
��
��
7�����������������=��������ͬ�¶��£������ʵ���Ũ�ȵ�������������Һ��pH�ɴ�С������˳��Ϊ
c��a��b��d
c��a��b��d
������ţ�a��NH4HCO3 b��NH4A c����NH4��2CO3 d��NH4Cl��
��������1�����ݸ�˹������дĿ���Ȼ�ѧ����ʽ��
��2������ͼ��֪����Ӧ�����������������������������÷�ӦΪ���ȷ�Ӧ�������¶�ƽ�����淴Ӧ�����ƶ����ݴ˽��
����ͼ��֪����Ӧ����B��A��ȣ��ı䷴Ӧ���̣�Ӧ��ʹ�ô�����
��3����ѧƽ�ⳣ������ָ��һ���¶��£����淴Ӧ�ﵽƽ��ʱ��������Ũ�ȵĻ�ѧ���������ݵij˻����Ը���Ӧ��Ũ�ȵĻ�ѧ���������ݵij˻����õı�ֵ���ݴ���д��ע���Ӧ���й������ʲμ�ʱ������ġ�Ũ�ȡ���Ϊ��������ƽ�ⳣ������ʽ�У��Ͳ�д�����Ũ�ȣ�ϡ��Һ�е�ˮ����Ũ�ȿɲ�д�����ڷ�ˮ��Һ�еķ�Ӧ���ܼ���Ũ��ͬ���dz�����
K1000����K1200����������ӦΪ���ȷ�Ӧ��������ϵ�¶ȣ�ƽ��������Ӧ�����ƶ���������������������ܵ����ʵ������䣬�ݴ��жϣ�
��4��0.1mol?L-1 HA��Һ��0.1mol?L-1 NaOH��Һ�������ϣ���Ϻ���Һ����ı仯���Բ��ƣ�����û��Һ��pH=8����˵��HA�����ᣬ�����������ӵ��δٽ�ˮ���룬
�ٸ�������Һ��pH����ˮ�������c��OH-��������c��NaOH����ˮ�����ӻ���������ˮ��������Ũ�ȣ���Һ��������Ũ�ȵ���ˮ�������c��OH-�����ݴ˽��
�ڸ�������֪��HA�����Ա�̼��ǿ��NH4A��ҺΪ���ԣ�˵��笠����Ӻ��������ˮ��̶���ͬ���ɴ˵�֪笠�����ˮ��̶�С��̼������ӣ����ԣ�NH4��2CO3����Һ�ʼ��ԣ�
����笠����ӵ�ˮ��̶��ж���Һ����ԵĴ�С����ҺŨ��Խϡ���ε�ˮ��̶�Խ��
��2������ͼ��֪����Ӧ�����������������������������÷�ӦΪ���ȷ�Ӧ�������¶�ƽ�����淴Ӧ�����ƶ����ݴ˽��
����ͼ��֪����Ӧ����B��A��ȣ��ı䷴Ӧ���̣�Ӧ��ʹ�ô�����
��3����ѧƽ�ⳣ������ָ��һ���¶��£����淴Ӧ�ﵽƽ��ʱ��������Ũ�ȵĻ�ѧ���������ݵij˻����Ը���Ӧ��Ũ�ȵĻ�ѧ���������ݵij˻����õı�ֵ���ݴ���д��ע���Ӧ���й������ʲμ�ʱ������ġ�Ũ�ȡ���Ϊ��������ƽ�ⳣ������ʽ�У��Ͳ�д�����Ũ�ȣ�ϡ��Һ�е�ˮ����Ũ�ȿɲ�д�����ڷ�ˮ��Һ�еķ�Ӧ���ܼ���Ũ��ͬ���dz�����
K1000����K1200����������ӦΪ���ȷ�Ӧ��������ϵ�¶ȣ�ƽ��������Ӧ�����ƶ���������������������ܵ����ʵ������䣬�ݴ��жϣ�
��4��0.1mol?L-1 HA��Һ��0.1mol?L-1 NaOH��Һ�������ϣ���Ϻ���Һ����ı仯���Բ��ƣ�����û��Һ��pH=8����˵��HA�����ᣬ�����������ӵ��δٽ�ˮ���룬
�ٸ�������Һ��pH����ˮ�������c��OH-��������c��NaOH����ˮ�����ӻ���������ˮ��������Ũ�ȣ���Һ��������Ũ�ȵ���ˮ�������c��OH-�����ݴ˽��
�ڸ�������֪��HA�����Ա�̼��ǿ��NH4A��ҺΪ���ԣ�˵��笠����Ӻ��������ˮ��̶���ͬ���ɴ˵�֪笠�����ˮ��̶�С��̼������ӣ����ԣ�NH4��2CO3����Һ�ʼ��ԣ�
����笠����ӵ�ˮ��̶��ж���Һ����ԵĴ�С����ҺŨ��Խϡ���ε�ˮ��̶�Խ��
����⣺��1����Fe��s��+
O2��g��=FeO��s����H=-272.0kJ?mol-1
��2Al��s��+
O2��g��=Al2O3��s����H=-1675.7kJ?mol-1
���ݸ�˹���ɢ�-�١�3�õ��Ȼ�ѧ����ʽΪ��2Al��s��+3FeO��s���TAl2O3��s��+3Fe��s����H=-859.7 kJ?mol-1
�ʴ�Ϊ��2Al��s��+3FeO��s���TAl2O3��s��+3Fe��s����H=-859.7 kJ?mol-1��
��2������ͼ��֪����Ӧ�����������������������������÷�ӦΪ���ȷ�Ӧ�������¶�ƽ�����淴Ӧ�����ƶ�����Ӧ���ת���ʼ�С��
�ʴ�Ϊ��������С��
����ͼ��֪����Ӧ����B��A��ȣ��ı䷴Ӧ���̣�Ӧ��ʹ�ô�����
�ʴ�Ϊ��D��
��3��Na2SO4��S��+4H2��g���TNa2S��s��+4H2O��g����ƽ�ⳣ������ʽk=
��
K1000����K1200����������ӦΪ���ȷ�Ӧ��������ϵ�¶ȣ�ƽ��������Ӧ�����ƶ���������������������ܵ����ʵ������䣬�ʻ�������ƽ����Է�������������
�ʴ�Ϊ��
������
��4��������Һ��c��OH-��=
=10-6 mol/L��0.1mol?L-1NaOH��Һ��c��H+��������ˮ�����������������Ũ�ȣ���c��H+��=cˮ������OH-��=
=10-13 mol/L�����Ի��Һ����ˮ�������OH-Ũ����0.1mol?L-1NaOH��Һ����ˮ�������OH-Ũ��֮��=10-6 mol/L��10-13 mol/L=107��1��
�ʴ�Ϊ��107��1��
�ڽ�HA��Һ�ӵ�Na2CO3��Һ��������ų���˵��HA�����Ա�̼���ǿ��NH4A��ҺΪ���ԣ�˵����ͬ�����£���ˮ��HA�ĵ���̶���ͬ�����ԣ�NH4��2CO3��笠����ӵ�ˮ��̶�С��̼������ӵ�ˮ��̶ȣ�������Һ��pH��7��
NH4Cl��ǿ�������Σ�笠�����ˮ�����Һ�����ԣ�
NH4A��Һ���������ӵ�ˮ��̶���ȣ�������Һ�����ԣ���Һ��pHֵ�����Ȼ�泥�
NH4HCO3��Һ��笠����ӵ�ˮ��̶�С��̼��������ӵ�ˮ��̶ȣ���Һ�ʼ��ԣ�
��NH4��2CO3��Һ��̼�����ˮ��̶ȸ���pHֵ���
�ʴ�Ϊ������c��a��b��d��
| 1 |
| 2 |
��2Al��s��+
| 3 |
| 2 |
���ݸ�˹���ɢ�-�١�3�õ��Ȼ�ѧ����ʽΪ��2Al��s��+3FeO��s���TAl2O3��s��+3Fe��s����H=-859.7 kJ?mol-1
�ʴ�Ϊ��2Al��s��+3FeO��s���TAl2O3��s��+3Fe��s����H=-859.7 kJ?mol-1��
��2������ͼ��֪����Ӧ�����������������������������÷�ӦΪ���ȷ�Ӧ�������¶�ƽ�����淴Ӧ�����ƶ�����Ӧ���ת���ʼ�С��
�ʴ�Ϊ��������С��
����ͼ��֪����Ӧ����B��A��ȣ��ı䷴Ӧ���̣�Ӧ��ʹ�ô�����
�ʴ�Ϊ��D��
��3��Na2SO4��S��+4H2��g���TNa2S��s��+4H2O��g����ƽ�ⳣ������ʽk=
| c4(H2O) |
| c4(H2) |
K1000����K1200����������ӦΪ���ȷ�Ӧ��������ϵ�¶ȣ�ƽ��������Ӧ�����ƶ���������������������ܵ����ʵ������䣬�ʻ�������ƽ����Է�������������
�ʴ�Ϊ��
| c4(H2O) |
| c4(H2) |
��4��������Һ��c��OH-��=
| 10-14 |
| 10-8 |
| 10-14 |
| 0.1 |
�ʴ�Ϊ��107��1��
�ڽ�HA��Һ�ӵ�Na2CO3��Һ��������ų���˵��HA�����Ա�̼���ǿ��NH4A��ҺΪ���ԣ�˵����ͬ�����£���ˮ��HA�ĵ���̶���ͬ�����ԣ�NH4��2CO3��笠����ӵ�ˮ��̶�С��̼������ӵ�ˮ��̶ȣ�������Һ��pH��7��
NH4Cl��ǿ�������Σ�笠�����ˮ�����Һ�����ԣ�
NH4A��Һ���������ӵ�ˮ��̶���ȣ�������Һ�����ԣ���Һ��pHֵ�����Ȼ�泥�
NH4HCO3��Һ��笠����ӵ�ˮ��̶�С��̼��������ӵ�ˮ��̶ȣ���Һ�ʼ��ԣ�
��NH4��2CO3��Һ��̼�����ˮ��̶ȸ���pHֵ���
�ʴ�Ϊ������c��a��b��d��
�����������ۺ��Խϴ��漰�Ȼ�ѧ����ʽ����д����ѧƽ�ⳣ����ƽ���ƶ����������Һ�йؼ��㡢����ˮ��ȣ��Ѷ��еȣ��Ƕ�ѧ���ۺ������Ŀ��飬��5��Ϊ�״��㣬�ж�HA��̼������ǿ���ǹؼ���

��ϰ��ϵ�д�
 ��ѧ����ͬ����ϰϵ�д�
��ѧ����ͬ����ϰϵ�д� ��ǰ�κ�ͬ����ϰϵ�д�
��ǰ�κ�ͬ����ϰϵ�д� ����С��ҵϵ�д�
����С��ҵϵ�д�
�����Ŀ