��Ŀ����
�������糧�ͷų������ĵ������NOx������������Ͷ�����̼���������ɻ�����Ⱦ����ȼú����������̼������ȴ�������ʵ����ɫ���������ܼ��š��������õ�Ŀ�ģ�
��1����̼����CO2ת��Ϊ�״����Ȼ�ѧ����ʽΪ��CO2��g��+3H2��g��?CH3OH��g��+H2O��g����H3
��ȡ��ݵ������CO2��H2�Ļ�����壨���ʵ���֮�Ⱦ�Ϊ1��3�����ֱ�����¶Ȳ�ͬ���ݻ���ͬ�ĺ����ܱ������У�����������Ӧ����Ӧ��ͬʱ���ü״�����������գ�CH3OH�� �뷴Ӧ�¶�T�Ĺ�ϵ������ͼ����ʾ��������CO2ת��Ϊ�״��ķ�Ӧ�ġ�H3

����ͼ�����ں����ܱ������У�ѹǿΪP1ʱH2�����������ʱ��t�ı仯���ߣ����ڸ�ͼ�л����÷�Ӧ��P2��P2��P1��ʱ��H2���������ʱ��t�ı仯����
 ��
��
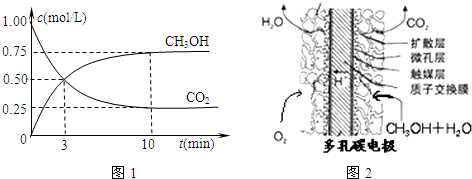
��2����һ���º����ܱ������г���1mol CO2��3mol H2������������Ӧ�����CO2��CH3OH��g�������ʵ���Ũ����ʱ��仯��ͼ����ʾ��
�Իش�0��10min�ڣ�������ƽ����Ӧ����Ϊ
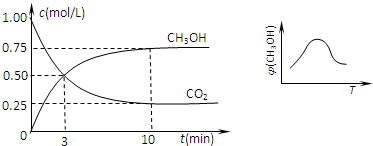
��3������ij���������н���������������һ�����İ�����������Ӧ����������狀�����淋Ļ������Ϊ����Ʒ���ʣ�����100mlһ�����ʵ���Ũ�ȵ��������Һ���貣���������ձ�����ͷ�ι���裺
��1����̼����CO2ת��Ϊ�״����Ȼ�ѧ����ʽΪ��CO2��g��+3H2��g��?CH3OH��g��+H2O��g����H3
��ȡ��ݵ������CO2��H2�Ļ�����壨���ʵ���֮�Ⱦ�Ϊ1��3�����ֱ�����¶Ȳ�ͬ���ݻ���ͬ�ĺ����ܱ������У�����������Ӧ����Ӧ��ͬʱ���ü״�����������գ�CH3OH�� �뷴Ӧ�¶�T�Ĺ�ϵ������ͼ����ʾ��������CO2ת��Ϊ�״��ķ�Ӧ�ġ�H3
��
��
0�����������������=����
����ͼ�����ں����ܱ������У�ѹǿΪP1ʱH2�����������ʱ��t�ı仯���ߣ����ڸ�ͼ�л����÷�Ӧ��P2��P2��P1��ʱ��H2���������ʱ��t�ı仯����


��2����һ���º����ܱ������г���1mol CO2��3mol H2������������Ӧ�����CO2��CH3OH��g�������ʵ���Ũ����ʱ��仯��ͼ����ʾ��
�Իش�0��10min�ڣ�������ƽ����Ӧ����Ϊ
0.225mol/��L?min��
0.225mol/��L?min��
�����¶��£���Ӧ��ƽ�ⳣ��Ϊ5.33
5.33
��������������Ч���֣���10min������������ٳ���1mol CO2��3mol H2�����ٴδﵽƽ��ʱCH3OH��g��������������
���
�������١����䣩����3������ij���������н���������������һ�����İ�����������Ӧ����������狀�����淋Ļ������Ϊ����Ʒ���ʣ�����100mlһ�����ʵ���Ũ�ȵ��������Һ���貣���������ձ�����ͷ�ι���裺
��������100mL����ƿ
��������100mL����ƿ
����������1���١���ͼ��֪��ߵ㷴Ӧ����ƽ�⣬����ƽ����¶�Խ�ߣ��գ�CH3OH��ԽС������ƽ�����淴Ӧ���У��ݴ��жϣ�
�ڡ�����ѹǿ����Ӧ���ʼӿ죬����ƽ��ʱ�����̣�����ѹǿƽ��������Ӧ�ƶ���ƽ��ʱƽ��ʱ����������������ͣ��ݴ���ͼ��
��2����ͼ��֪��0��10min�ڶ�����̼��Ũ�ȱ仯Ϊ1mol/L-0.25mol/L=0.75mol/L������c=
����v��CO2���������ø����ʵķ�Ӧ����֮�ȵ��ڼ�����֮�ȼ���v��H2����
��������ʽ����ƽ��ʱ����ֵ�ƽ��Ũ�ȣ�����ƽ�ⳣ������ʽ����ƽ�ⳣ����
��10min������������ٳ���1mol CO2��3mol H2����ЧΪ����ѹǿ��ƽ��������Ӧ�ƶ���CH3OH��g���������������
��3������100mlһ�����ʵ���Ũ�ȵ��������Һ���貣�������У��ձ�����������100mL����ƿ����ͷ�ιܣ�
�ڡ�����ѹǿ����Ӧ���ʼӿ죬����ƽ��ʱ�����̣�����ѹǿƽ��������Ӧ�ƶ���ƽ��ʱƽ��ʱ����������������ͣ��ݴ���ͼ��
��2����ͼ��֪��0��10min�ڶ�����̼��Ũ�ȱ仯Ϊ1mol/L-0.25mol/L=0.75mol/L������c=
| ��c |
| ��t |
��������ʽ����ƽ��ʱ����ֵ�ƽ��Ũ�ȣ�����ƽ�ⳣ������ʽ����ƽ�ⳣ����
��10min������������ٳ���1mol CO2��3mol H2����ЧΪ����ѹǿ��ƽ��������Ӧ�ƶ���CH3OH��g���������������
��3������100mlһ�����ʵ���Ũ�ȵ��������Һ���貣�������У��ձ�����������100mL����ƿ����ͷ�ιܣ�
����⣺��1���١���ͼ��֪��ߵ㷴Ӧ����ƽ�⣬��ƽ����¶�Խ�ߣ��գ�CH3OH��ԽС��˵�������¶�ƽ�����淴Ӧ���У������¶�ƽ�����ȷ�����У��淴ӦΪ���ȷ�Ӧ��������ӦΪ���ȷ�Ӧ������H3��0���ʴ�Ϊ������
������ѹǿ����Ӧ���ʼӿ죬����ƽ��ʱ�����̣�����ѹǿƽ��������Ӧ�ƶ���ƽ��ʱƽ��ʱ����������������ͣ�����P2��P2��P1��ʱ��H2���������ʱ��t�ı仯����Ϊ�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��2����ͼ��֪��10minʱ����Ӧ�Ѿ���ƽ�⣬��c��CO2��=1.00mol/L-0.25mol/L=0.75mol/L������10min�ڣ�v��CO2��=
=0.075mol/��L?min������Ӧ����֮�ȵ��ڼ�����֮�ȣ���v��H2��=3v��CO2��=3��0.075mol/��L?min��=0.225mol/��L?min����
��ʼCO2��Ũ��Ϊ1mol/L�������������Ϊ
=1L��H2����ʼŨ��Ϊ
=3mol/L����
CO2��g��+3H2��g��?CH3OH��g��+H2O��g��
��ʼ��mol/L����1 3 0 0
�仯��mol/L����0.75 2.25 0.75 0.75
ƽ�⣨mol/L����0.25 0.75 0.75 0.75
�ʸ��¶���ƽ�ⳣ��k=
=5.33��
��10min������������ٳ���1mol CO2��3mol H2����ЧΪ����ѹǿ��ƽ��������Ӧ�ƶ���CH3OH��g���������������
�ʴ�Ϊ��0.225mol/��L?min����5.33�����
��3������100mlһ�����ʵ���Ũ�ȵ��������Һ���貣�������У��ձ�����������100mL����ƿ����ͷ�ιܣ��ʻ���Ҫ�IJ�������Ϊ����������100mL����ƿ��
�ʴ�Ϊ����������100mL����ƿ��
������ѹǿ����Ӧ���ʼӿ죬����ƽ��ʱ�����̣�����ѹǿƽ��������Ӧ�ƶ���ƽ��ʱƽ��ʱ����������������ͣ�����P2��P2��P1��ʱ��H2���������ʱ��t�ı仯����Ϊ��
 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
����2����ͼ��֪��10minʱ����Ӧ�Ѿ���ƽ�⣬��c��CO2��=1.00mol/L-0.25mol/L=0.75mol/L������10min�ڣ�v��CO2��=
| 0.75mol/L |
| 10min |
��ʼCO2��Ũ��Ϊ1mol/L�������������Ϊ
| 1mol |
| 1mol/L |
| 3mol |
| 1L |
CO2��g��+3H2��g��?CH3OH��g��+H2O��g��
��ʼ��mol/L����1 3 0 0
�仯��mol/L����0.75 2.25 0.75 0.75
ƽ�⣨mol/L����0.25 0.75 0.75 0.75
�ʸ��¶���ƽ�ⳣ��k=
| 0.75��0.75 |
| 0.25��0.753 |
��10min������������ٳ���1mol CO2��3mol H2����ЧΪ����ѹǿ��ƽ��������Ӧ�ƶ���CH3OH��g���������������
�ʴ�Ϊ��0.225mol/��L?min����5.33�����
��3������100mlһ�����ʵ���Ũ�ȵ��������Һ���貣�������У��ձ�����������100mL����ƿ����ͷ�ιܣ��ʻ���Ҫ�IJ�������Ϊ����������100mL����ƿ��
�ʴ�Ϊ����������100mL����ƿ��
������������ȼú����������̼������ȴ���Ϊ���壬���黯ѧƽ���й�ͼ��Ӧ���ʼ��㡢ƽ�ⳣ�����㡢Ӱ�컯ѧƽ������ء���Һ���Ƶȣ��Ѷ��еȣ�������ѧ�����������������������ע�⣨1������ͼ����ߵ��������ϵĸ��㶼��ƽ��״̬��

��ϰ��ϵ�д�
�����Ŀ
 ��2011?��ƽ��ģ���������糧�ͷų������ĵ������NOx������������Ͷ�����̼���������ɻ�����Ⱦ����ȼú���������������������̼�ȴ�������ʵ����ɫ���������ܼ��š��������õ�Ŀ�ģ�
��2011?��ƽ��ģ���������糧�ͷų������ĵ������NOx������������Ͷ�����̼���������ɻ�����Ⱦ����ȼú���������������������̼�ȴ�������ʵ����ɫ���������ܼ��š��������õ�Ŀ�ģ�
 NH3?H2O+H+
NH3?H2O+H+