��Ŀ����
����Ŀ���Ӻ�ˮ�п�����ȡ�ܶ����õ����ʣ�����Ӻ�ˮ�������õ���±ˮ�п�����ȡ�⡣����̿�������ǹ�ҵ���ķ���֮һ�����������£�
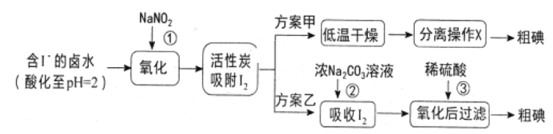
������ʾ����pH=2ʱ��NaNO2��Һֻ�ܽ�I-����ΪI2,ͬʱ����NO;
��I2+5Cl2+6H2O=2HIO3+10HCl;
��5SO32-+2IO3-+2H+=I2+5SO42-+H2O;
����I2�ڼ�����Һ�з�Ӧ����I-��IO3-��
(1)��Ӧ�ٵ����ӷ���ʽ_________________________________________��
(2)�������У�����I2�����ԣ��������X��������________________��
(3)��֪����Ӧ����ÿ����3molI2ת��5mol���ӣ������ӷ���ʽ��_______________��
(4)Cl2������KMnO4�ȶ��dz��õ�ǿ�����������ù���������±ˮ�е�I-ȴѡ���˼۸�ϸߵ�NaNO2,ԭ����_______________��
(5)�������У���֪��Ӧ�۹��˺���Һ���Դ���������I2��I-��IO3-����ֱ������Һ�е�I-��IO3-����ʵ�鷽������������ʵ���пɹ�ѡ����Լ���ϡH2SO4��������Һ��Fe2(SO4)3��Һ��Na2SO3��Һ
A����Һ��CCl4�����ȡ����Һ��ֱ��ˮ���õ�����Һ���鲻���ⵥ�ʴ��ڡ�
B��_____________________________________________________________��
(6)ijѧ���ƻ���12 mol��L��1��Ũ��������0.10 mol��L��1��ϡ����450 mL���ش��������⣺ʵ������У�����ʹ�õ���________(����ĸ)��
A��������ƽ��B����Ͳ��C������ƿ��D��250 mL�ձ� E����ͷ�ιܡ�F. 500 mL�Լ�ƿ
(7)�����������п�ʹ�õ����⣬��ȱ�ٵ�������______���ڸ�ʵ���е���;��________________��
(8)��ȡŨ��������Ϊ______ mL��Ӧѡ�õ���Ͳ���Ϊ________��
(9)����ʱӦѡ�õ�����ƿ���Ϊ______________��
���𰸡�2NO2-+2 I-+4H+ =I2+2NO+2H2O����������ȣ��������ᾧ���������ᾧ���۷֣�3I2+3CO32-=5I-+IO3-+3CO2 ����3I2+6CO32-+3H2O=5I-+IO3-+6HCO3-�����������Ը�����صȶ��dz��õ�ǿ�����������������I2(���������ƽ��ܰѵ����������ɵⵥ�ʣ���˼�Լ���)��ˮ��ȡ������Һ���Թ��У����뼸�ε�����Һ���μ�Fe2(SO4)3��Һ������Һ������˵����Һ�к���I��������ˮ����ȡ������Һ���Թ��У����뼸�ε�����Һ���������ữ���μ�Na2SO3��Һ������Һ������˵����Һ�к���IO3��A������ϡ��Ũ����ʱ��������ã�ʹ��Һ��Ͼ��ȣ����ձ���ϡ�͵���Һת�Ƶ�����ƿ��ʱ����������4.210 mL500 mL
��������
(1)�������ƾ���������,�����Ӿ��л�ԭ��,���������¶��߷���������ԭ��Ӧ����һ�������͵��ˮ,���ӷ�Ӧ����ʽΪ2NO2-+2I-+4H+ =I2+2NO+2H2O����ȷ�𰸣�2NO2-+2 I-+4H+ =I2+2NO+2H2O��
(2)��������,�������У��������XΪ��������ȡ������ᾧ����ˣ�������ȷ����:��������ȡ������ᾧ��
(3)��Ӧ����ÿ����3molI2ת��5mol���ӣ�˵������I-��IO3-,������ӷ���ʽΪ: 3I2+3CO32-=5I-+IO3-+3CO2 ����3I2+6CO32-+3H2O=5I-+IO3-+6HCO3-������ȷ�𰸣�3I2+3CO32-=5I-+IO3-+3CO2 ����3I2+6CO32-+3H2O=5I-+IO3-+6HCO3-����
(4)���������Ը�����صȶ��dz��õ�ǿ������������������ⵥ�ʣ����������ƽ��ܰѵ���������Ϊ�ⵥ�ʣ��ʸù���������±ˮ�еĵ����ӣ�ѡ���˼۸�ϸߵ��������ƣ���ˣ�������ȷ���ǣ����������Ը�����صȶ��dz��õ�ǿ�����������������I2(���������ƽ��ܰѵ����������ɵⵥ�ʣ���˼�Լ���)��
(5)������Һ�еĵ����ӡ���������ӣ����õ����ӱ��������ɵⵥ�ʼ��飬��������ӱ���ԭ���ɵⵥ�ʼ��飬����Ϊ����ˮ��ȡ������Һ���Թ���,���뼸�ε�����Һ,�μ� Fe2(SO4)3��Һ,��,��Һ����,˵����Һ�к���I������ȡ��ˮ��ȡ������Һ���Թ���,���뼸�ε�����Һ,�������ữ,�μ�Na2SO3��Һ,����Һ������˵����Һ�к���IO3�� ����ȷ�𰸣���ˮ��ȡ������Һ���Թ��У����뼸�ε�����Һ���μ�Fe2(SO4)3��Һ������Һ������˵����Һ�к���I��������ˮ����ȡ������Һ���Թ��У����뼸�ε�����Һ���������ữ���μ�Na2SO3��Һ������Һ������˵����Һ�к���IO3�� ��
��6����Ũ��Һ����ϡ��Һʱ��Ӧѡ����Ͳ������������ƽ����ȷ�𰸣�A��
��7������һ��Ũ�ȵ�ϡ������Һ��ͨ����6����֪����ȱ�ٵ������Dz�����������ϡ��Ũ����ʱ��������ã�ʹ��Һ��Ͼ��ȣ����ձ���ϡ�͵���Һת�Ƶ�����ƿ��ʱ���������ã���ȷ�𰸣���������ϡ��Ũ����ʱ��������ã�ʹ��Һ��Ͼ��ȣ����ձ���ϡ�͵���Һת�Ƶ�����ƿ��ʱ���������á�
��8������ȡŨ��������ΪV mL������ϡ��ǰ��HCl���ʵ����غ㽨����ϵʽ��12 mol��L��1��V��10-3��0.10 mol��L��1��500 ��10-3��V��4.2 mL��������Ͳ�Ĺ��ѡȡ10 mL��Ͳ����ȷ�𰸣�4.2 ��10 mL��
��9������ϡ����450 mL������û�д��ֹ�������ƿ��������500 mL������ƿ����ȷ�𰸣�500 mL��



