��Ŀ����
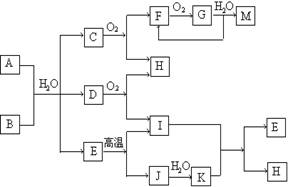
��֪��AΪ��ʽ�Σ�BΪij�����������Ԫ�صĻ��������B�Ļ�ѧʽ�й���32�����ӣ����³�ѹ��C��D��F��G��I������̬����G�ʺ���ɫ�����������Ϊ��ɫ�������ʵ���A��B��������ˮ��ֻ����ǡ����ȫ��Ӧ��ͼ�з�Ӧ�������������⣩������ȥ��
��1��д��B�ĵ���ʽ
��2��д�����з�Ӧ�����ӷ���ʽ��
����A��Һ�м���M
����A��Һ�м������NaOH��Һ��������
��3��C�����ڴ����а���ȼ�գ�����ΪH��һ�ֵ��ʣ�д���÷�Ӧ�Ļ�ѧ����ʽ��
��4��д��A��B��Ӧ�Ļ�ѧ����ʽ ��
��1��
��2����HCO3- +H+ = H2O +CO2��
��NH4+ +HCO3- +2OH-�� NH3��+2H2O+CO32-
��3�� 4NH3 +3O 2 =2N2 +6H2O
2 =2N2 +6H2O
��4��CaC2 + NH4HCO3 = NH3��+C2H2��+CaCO3��
����

��ϰ��ϵ�д�
�����Ŀ
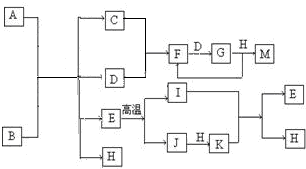 ��֪��AΪ��ʽ�Σ�BΪij�����������Ԫ�صĻ��������B�Ļ�ѧʽ�й���36�����ӣ����³�ѹ��C��D��F��G��I������̬����G�ʺ���ɫ�����������Ϊ��ɫ��H�ڳ���������ɫҺ�壮�����ʵ���A��B��������H��ֻ����ǡ����ȫ��Ӧ��ͼ�з�Ӧ�������������⣩������ȥ��
��֪��AΪ��ʽ�Σ�BΪij�����������Ԫ�صĻ��������B�Ļ�ѧʽ�й���36�����ӣ����³�ѹ��C��D��F��G��I������̬����G�ʺ���ɫ�����������Ϊ��ɫ��H�ڳ���������ɫҺ�壮�����ʵ���A��B��������H��ֻ����ǡ����ȫ��Ӧ��ͼ�з�Ӧ�������������⣩������ȥ��