��Ŀ����
��֪A��B��C��D�Ƕ������е����ַǽ���Ԫ�أ����ǵ�ԭ������������������Ԫ�ص�ԭ������������֮��Ϊ16��A��B��C��D֮������γɺ���10�����ӻ�18�����ӵĻ������A��D��ɵĻ������ڳ����³�Һ̬��
��1��A��C����Ԫ����ɵ�10�����ӻ�����ķ��ӵĿռ乹����_________������________����ԡ��Ǽ��ԣ����ӡ�
��2��B�����ڸ����¿ɴ�A��D��ɵ�ij�ֻ��������û���A��ͬʱ���ɻ�����BD���÷�Ӧ�Ļ�ѧ����ʽΪ____________________��
��3��A��C��D����Ԫ��ԭ�Ӱ뾶��С�����˳���ǣ���Ԫ�ط��ű�ʾ��__________����A��C�� A��D���ɵ����ַ��ӣ����H+������________ǿ���ѧʽ���������ӷ���ʽ��ʾ��һ��ʵ��__________��
��4��A��B��D����Ԫ���γɵľ���18�����ӵĻ�����Ľṹ��ʽΪ__________��
��5��������Ԫ�ؿ����ԭ�Ӹ�����Ϊ5:1:1:2����A��B��C��D˳�Ļ����������Ϊ_________��
��1��A��C����Ԫ����ɵ�10�����ӻ�����ķ��ӵĿռ乹����_________������________����ԡ��Ǽ��ԣ����ӡ�
��2��B�����ڸ����¿ɴ�A��D��ɵ�ij�ֻ��������û���A��ͬʱ���ɻ�����BD���÷�Ӧ�Ļ�ѧ����ʽΪ____________________��
��3��A��C��D����Ԫ��ԭ�Ӱ뾶��С�����˳���ǣ���Ԫ�ط��ű�ʾ��__________����A��C�� A��D���ɵ����ַ��ӣ����H+������________ǿ���ѧʽ���������ӷ���ʽ��ʾ��һ��ʵ��__________��
��4��A��B��D����Ԫ���γɵľ���18�����ӵĻ�����Ľṹ��ʽΪ__________��
��5��������Ԫ�ؿ����ԭ�Ӹ�����Ϊ5:1:1:2����A��B��C��D˳�Ļ����������Ϊ_________��
��1�� ����������
��2��
��3��H��O��N ��NH3
NH3+H3O+==NH4++H2O
��4��CH3OH
��5�������
��2��

��3��H��O��N ��NH3
NH3+H3O+==NH4++H2O
��4��CH3OH
��5�������

��ϰ��ϵ�д�
�����Ŀ
��֪A��B��C��D��ԭ��������������Ķ���������Ԫ�أ�A��B��C�ֱ��ڲ�ͬ���ڣ�A��Cͬ���壬B��һ�ֵ�����ʹ�����ǵ�ľ����ȼ��A��C��D����ԭ�ӵ�����������֮��Ϊ6������˵������ȷ���ǣ�������
| A��A��ԭ�Ӱ뾶��B��С | B��B��C�γɵĻ�����ֻ��һ�� | C��C�ڻ������г�+1�� | D��D���ʵľ���������뵼����� |
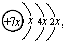 ��B��ͬ������ԭ�ӵ�һ��������С��Ԫ�أ�Cԭ�ӵ������������δ�ɶԵ��ӣ�E�������ճ���������õĽ�����
��B��ͬ������ԭ�ӵ�һ��������С��Ԫ�أ�Cԭ�ӵ������������δ�ɶԵ��ӣ�E�������ճ���������õĽ�����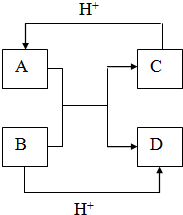 ��֪A��B��C��D����ѧ��ѧ�г��������ֲ�ͬ��������֮�������ͼ��ʾ��ת����ϵ��
��֪A��B��C��D����ѧ��ѧ�г��������ֲ�ͬ��������֮�������ͼ��ʾ��ת����ϵ��