��Ŀ����
12����A��B��C��D��E 5��Ԫ�أ����ǵĺ˵�������������Ҷ�С��20������C��E �ǽ���Ԫ�أ�A��E��ͬһ�壬����ԭ�ӵ����������Ų�Ϊns1��B��DҲ��ͬһ�壬����ԭ��������p�ܼ���������s�ܼ���������������Cԭ��������ϵ���������Dԭ��������ϵ�������һ�룮��ش��������⣮��1��A��H��B��Na��C��Al��E��K��
��2��B�����ڱ���λ��p����
��3��д��CԪ�ػ�̬ԭ�ӵĵ����Ų�ʽ1s22s22p63s23p1��
��4���ù����ʾʽ��ʾDԪ��ԭ�ӵļ۵��ӹ���
 ��
��
���� A��B��C��D��E���ֶ�����Ԫ�أ����ǵĺ˵�������������Ҷ�С��20��B��Dͬ�壬��ԭ��������p�ܼ���������s�ܼ�����������������Χ�����Ų�Ϊns2np4����BΪOԪ�ء�DΪSԪ�أ�CԪ��ԭ�ӵ�������������DԪ��ԭ��������������һ�룬��Cԭ������������Ϊ6��$\frac{1}{2}$=3����CΪAlԪ��Ԫ�أ�A��E���������Ų�Ϊns1�����ߴ��ڢ�A�壬Eԭ������������Ԫ�أ�EΪKԪ�أ�ֻ��C��EΪ����Ԫ�أ���AΪHԪ�أ��ݴ˽��
��� �⣺A��B��C��D��E���ֶ�����Ԫ�أ����ǵĺ˵�������������Ҷ�С��20��B��Dͬ�壬��ԭ��������p�ܼ���������s�ܼ�����������������Χ�����Ų�Ϊns2np4����BΪOԪ�ء�DΪSԪ�أ�CԪ��ԭ�ӵ�������������DԪ��ԭ��������������һ�룬��Cԭ������������Ϊ6��$\frac{1}{2}$=3����CΪAlԪ��Ԫ�أ�A��E���������Ų�Ϊns1�����ߴ��ڢ�A�壬Eԭ������������Ԫ�أ�EΪKԪ�أ�ֻ��C��EΪ����Ԫ�أ���AΪHԪ�أ�
��1��������������֪��A��H��B��O��C��Al��E��K���ʴ�Ϊ��H��O��Al��K��
��2��BΪOԪ�أ���Χ�����Ų�Ϊ2s22p4�������ڱ���λ��p�����ʴ�Ϊ��p��
��3��CΪAlԪ�أ���̬ԭ�ӵĵ����Ų�ʽΪ1s22s22p63s23p1���ʴ�Ϊ��1s22s22p63s23p1��
��4��DΪSԪ�أ���Χ�����Ų�Ϊ3s23p4���ù����ʾʽ��ʾ�۵��ӹ���Ϊ�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
���� ���⿼��ṹ��λ�ù�ϵ��Ԫ�ص��ƶ��ǽ��Ĺؼ���ע��Ժ�������Ų����������գ�ּ�ڿ���ѧ���Ի���֪ʶ�����գ�

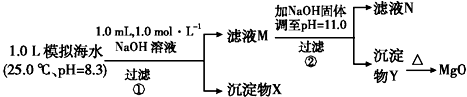
| ģ�⺣ˮ�е��� ��Ũ��/mol•L-1 | Na+ | Mg2+ | Ca2+ | Cl- | HCO3- |
| 0.439 | 0.050 | 0.011 | 0.560 | 0.001 |
ʵ������У�������Һ������䣮
KspCaCO3=4.96��10-9�� KspMgCO3=6.82��10-6 KspCa��OH��2=4.68��10-6
KspMg��OH��2=5.61��10-12
����˵������ȷ���ǣ�������
| A�� | ������XΪCaCO3��MgCO3 | |
| B�� | ��ҺM�д���Mg2+��Ca2+ | |
| C�� | ��ҺN�д���Ca2+��û��Mg2+ | |
| D�� | �����������Ϊ����4.2 g NaOH���壬������YΪ��Mg��OH��2��û��Ca��OH��2 |
| ���Ӿ��� | ԭ�Ӿ��� | ���Ӿ��� | |
| A | NaOH | Ar | SO2 |
| B | H2SO4 | ʯī | S |
| C | CH3COONa | ˮ�� | CH3CH3 |
| D | Ba��OH��2 | ���ʯ | ���� |
| A�� | A | B�� | B | C�� | C | D�� | D |
| ������ ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| 2 | �� | �� | ||||||
| 3 | �� | �� | �� | �� | �� | �� | �� | |
| 4 | �� | ⑪ | ⑫ |
��2������ЩԪ���У�����õĽ���Ԫ����K������õķǽ���Ԫ����F������õ�Ԫ����Ar��
��3������ЩԪ�ص�����������Ӧ��ˮ�����У�������ǿ����HClO4��������ǿ����KOH����Ϊ���������������Al��OH��3��
��4������ЩԪ���У�ԭ�Ӱ뾶��С����F��ԭ�Ӱ뾶������K��
��5���ڢ���ܣ���ѧ���ʽϻ��õ���Na��
| A�� | ����ĵ���ʽ�� | B�� | �״��ķ���ʽ��CH4O | ||
| C�� | ��ԭ�ӵ�ԭ�ӽṹʾ��ͼ�� | D�� | ����Ľṹ��ʽ��C3H8 |

 CH3COOC2H5+H2O
CH3COOC2H5+H2O ��ͼ��ʾװ�ã�c��d������ʯī�缫���ش��������⣺
��ͼ��ʾװ�ã�c��d������ʯī�缫���ش��������⣺