��Ŀ����
����Ŀ�������С³����ͷ�����ý�ͷ���棬���ŷ�������������ټӴ���������ԣ�Ȼ���������к��Ϲ������ɡ�С³�������ϵ�֪����ͷ��ʹ���������������²���CO2���壬�Ӷ�ʹ�������ɣ���ͬʱҲ��������ᡢ������л��ᡣ
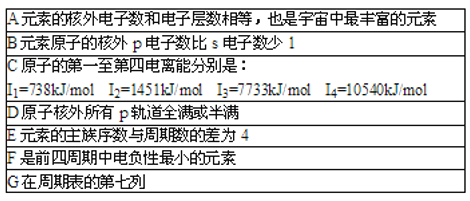
��1�����й��ڴ������ʶ��ȷ����___��
A�������ֽ� B��ˮ��Һ�Լ��� C�����ڼ� D�������ᷴӦ
��2��С³̽����Na2CO3����������ȣ�������NaHCO3��ԭ��
��Na2CO3��NaHCO3������Ϊ1gʱ������H+�����ʵ����������___��
����Na2CO3��NaHCO3Ϊ������������H+�����ʵ�����д��������̡�___
��3��С³ʵ�ʲ���ʱ���������ŷ��ò��ã������ڵ������٣�������ζ������ָ��С³��Na2CO3����NaHCO3���������棬�Ϲ������Ȼ��������������ͷ������NaHCO3������___��
��4��ijƷ�Ƹ������ɼ���˵������ͼ��ʾ��

������������-��-�����͵������������������Ƿ�ֹ���ɼ���������ʧЧ�������������Ƶ�������___��
���𰸡�BD Na2CO3 Na2CO3��1/53(0.0189)��NaHCO3��1/84(0.0119) ���ȷֽ����CO2���壬�Ӷ�ʹ�������ɣ������ᡢ������л��ᷴӦ��������ζ �ṩH+��NaHCO3��CaCO3��Ӧ����CO2���壬�Ӷ�ʹ��������
��������
��1������Ϊ̼���ƣ�����ǿ�������Σ�ˮ��Һ�Լ��ԣ������ᷴӦ���ɶ�����̼��ˮ��
��2����Na2CO3��NaHCO3����H+�����ʵ���֮��Ϊ![]() ��
��![]() =84��53��
=84��53��
��Na2CO3��NaHCO3����H+�����ʵ����ֱ�Ϊ![]() =0.0189mol��
=0.0189mol��![]() =0.0119mol��
=0.0119mol��
��3��NaHCO3�����ᡢ������л��ᷴӦ��������ζ�������ȷֽ����CO2���壬�Ӷ�ʹ�������ɣ�
��4�����������������ṩH+��NaHCO3��CaCO3��Ӧ����CO2���壬�Ӷ�ʹ�������ɡ�
��1������Ϊ̼���ƣ�����ǿ�������Σ�ˮ��Һ�Լ��ԣ������ᷴӦ���ɶ�����̼��ˮ������������ΪBD��
��2����Na2CO3��NaHCO3������Ϊ1gʱ������H+�����ʵ���֮��Ϊ![]() ��
��![]() =84��53��̼�������ĵ������Ӷࣻ
=84��53��̼�������ĵ������Ӷࣻ
��Na2CO3��NaHCO3����H+�����ʵ����ֱ�Ϊ![]() =0.0189mol��
=0.0189mol��![]() =0.0119mol��
=0.0119mol��
��3��NaHCO3�����ᡢ������л��ᷴӦ��������ζ�������ȷֽ����CO2���壬�Ӷ�ʹ�������ɣ�
��4�����������������ṩH+��NaHCO3��CaCO3��Ӧ����CO2���壬�Ӷ�ʹ�������ɡ�