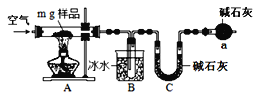
��Ŀ����
����Ŀ�������£���20.00 mL 0.1000mol��L1HA�еμ�0.1000mol��L1 NaOH��Һ����Һ��pH��NaOH��Һ����ı仯��ͼ����֪Ka(HA)=5��10-4��

����˵������ȷ���ǣ� ��
A.b��ʱ��HA�ĵ������A- ��ˮ�⣬��Һ������
B.�����������������a��ʱ��Һ��pH
C.������Һ�������仯��c��ʱpH����Ϊ8
D.�õζ���ѡ���̪��Ϊ�յ��жϵ�ָʾ��
���𰸡�B
��������
��Ka(HA)=5��10-4��֪��HAΪһԪ���ᣬHA��NaOH���ʵ���֮��Ϊ1:1ʱ����20mLNaOHʱ������ǡ����ȫ��Ӧ����Ӧ����Һ������ΪNaA���Լ��ԣ��ݴ˷������
A��b��ʱ��V(NaOH)=10mL��HA��Ӧ��һ�룬��Һ������Ϊ�����ʵ�����HA��NaA�� HA������������ӣ�A-ˮ�����OH-�����ݵ���ƽ�ⳣ����֪����̶ȴ���ˮ��̶ȣ�b����Һ�����ԣ���b��HA�ĵ������A- ��ˮ�⣬A��ȷ��
B��HA![]() H++A-��Ka(HA)=
H++A-��Ka(HA)= ��
��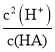 �����ԣ�c(H+)��
�����ԣ�c(H+)��![]() =
=![]() ��pH=-lg(
��pH=-lg(![]() )=2.5-lg
)=2.5-lg![]() ��B����
��B����
C��c��ʱ��HA��NaOHǡ����ȫ��Ӧ������ΪNaA��c(NaA)=0.05mol/L��A-+H2O![]() HA+OH-��Ka(HA)=
HA+OH-��Ka(HA)= ��
�� =
= �������ã�c2(H+)=
�������ã�c2(H+)=![]() =
=![]() =10-16��c(H+)=10-8��pH=8��C��ȷ��
=10-16��c(H+)=10-8��pH=8��C��ȷ��
D���ζ��յ��Լ��ԣ��÷�̪��ָʾ����D��ȷ��
��ѡB��

 Сѧ���AB��ϵ�д�
Сѧ���AB��ϵ�д� ABC����ȫ�ž�ϵ�д�
ABC����ȫ�ž�ϵ�д�����Ŀ��NaNO2�dz��õ�һ�ַ�����������+3�۵�N����һ���������ԡ�ijʵ��С���������·�Ӧ2NO2+2NaOH=NaNO3+NaNO2+H2O�Ʊ�NaNO2����̽�������ʡ�
I.�Ʊ�NaNO2
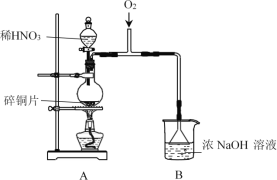
(1)A�з�����Ӧ�Ļ�ѧ����ʽ��______��
(2)B��ѡ��©�������ֱ���ܵ��ŵ���______��
(3)Ϊ����B���Ƶ�NaNO2����������ʵ�飺
��� | �Թ� | ���� | ���� |
�� | 2mLB����Һ | ��2mL0.1mol/LKI��Һ���μӼ��ε�����Һ | ������ |
�� | 2mLB����Һ | �μӼ���H2SO4��pH=5����2mL0.1mol/LKI��Һ���μӼ��ε�����Һ | ���� |
�� | 2mLH2O | �μӼ���H2SO4��pH=5����2mL0.1mol/LKI��Һ���μӼ��ε�����Һ | ������ |
ʵ��۵�Ŀ����_______��
(4)����Ϊ����3��ʵ����֤��B��һ����NaNO2�����貹��ʵ�飬������______��
II.̽��NaNO2������
װ�� | ���� | ���� |
| ȡ10mL1mol/LNaNO2��Һ���Լ�ƿ�У����뼸��H2SO4�ữ���ټ���10mL1mol��L-1FeSO4��Һ��Ѹ����������������ͨ������O2�� | i.��ҺѸ�ٱ�Ϊ��ɫ�� ii.��Һ��dz������ɫ���ݲ�������Һ�Ϸ�Ϊdz����ɫ�� iii.�����γ��غ�ɫ��Һ�� |
���ϣ�i.
ii.HNO2����Һ�в��ȶ����ֽ����NO��NO2���塣
(5)����i��Һ��Ϊ��ɫ��ԭ����______��
(6)��֪����ii��ɫ��Һ��dz������������Fe3+����Ӧ�����ӷ���ʽ��______��