��Ŀ����
��16�֣�ʵ������NH4HCO3��NaHSO3�ĸ����״����ij��ȤС��Ϊ�ⶨ����NH4HCO3�ĺ�������������ڻ�����м����ʵ�鷽�����ⶨ�������������ش��������⣺
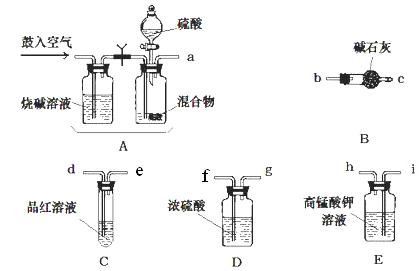
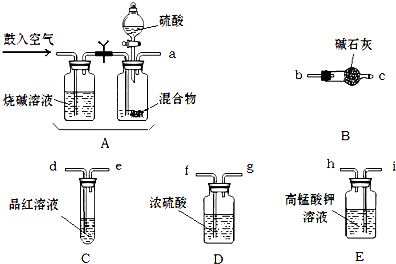
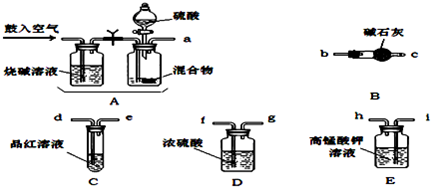
�� ��ѧС����Ҫѡ��������ҩƷ����������ʵ�顣��ͼ��ÿ������װ��ֻѡ����һ�Σ�����̨�ȹ̶�����δ������
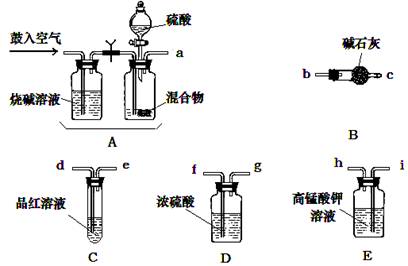
�밴�����������ҵķ�����������˳���ǣ��������Ľӿ���ĸa��b��������
a�� �� �� �� �� f ��g �� �� ��
�� ʵ�鿪ʼ����������ǰ��Aװ����Ҫ��������������� �������Aװ���ٴι�������������� ��
�� Eװ�õ������� ��
�� ʵ���У���Cƿ����Һ��ɫ����ⶨ������ܻ�ƫ ����ߡ��͡�����Ϊ��ȷ
��ʵ����Cƿ��Һ����ɫ����ȡ��Ʒ������Ϊm g��ʵ��ǰ E����װ a mol��L��1��KMnO4
��Һ�����V�������� mL��
�� ���������Ʒ����Ϊ13.1 g��������ú��ʯ������4.4 g����������NH4HCO3����
������Ϊ ��
�� �Ӷ����ⶨ��ȷ�Կ��ǣ�����װ�û�Ӧ����һ���ĸĽ��� ��
�����õ������ԭ��������H��1 C��12 N��14 O��16 Na��23 S��32
�� a��i��h��d��e��f��g��b��c
�� �ų�װ����ԭ�п����������ɵ�����ȫ���������װ�ã���ʹ��Ӧ������CO2
��SO2�ܱ���ȫ����Ҳ�� ��
�� ��ȥSO2���Է�ֹSO2�Բⶨ�����Ӱ��
�� �� 50m/��13a��
�� 60.3%
�� ��B֮���ٽ�һ����ֹ������ˮ�Ͷ�����̼����B��װ��
��������
�����������1������ʵ��ԭ�����������м������������̼�Ͷ����������壬�ø��������Һ��ȥ��������Ȼ��ͨ��Ʒ����Һ������������Ƿ������������ͨ��Ũ����������ü�ʯ�����ն�����̼���壬��ʯ�����ӵ�������Ϊ������̼���������������װ������˳����aihdefgbc����
��2��ʵ�鿪ʼ����������ǰ��Aװ����Ҫ�������Ŀ�����ų�ϵͳ�еĶ�����̼���壬������ٴι��������������ʹ���ɵ�����ȫ���������װ�á�
��3�����������Һ�����������ն����������塣
��4����Ʒ����Һ��ɫ��˵��������������û�г��������Լ�ʯ��������ƫ�ⶨ�����ƫ�ߡ����ݵ�ʧ������ȵö��������������ع�ϵΪ5SO2��2KMnO4����mg����ȫ�����������ƣ������ɶ�������m/104mol����Ҫ����������ʵ���Ϊm/260mol�����Ը��������Һ���������50m/13amL��
��5����ʯ������4.4g��������̼���ʵ���Ϊ0.1mol����̼Ԫ���غ㣬̼��������ʵ���ҲΪ0.1mol��̼���������Ϊ7.9g������̼�������������Ϊ60.3%��
��6����ʯ���������ͨ����ʯ�������տ����еĶ�����̼��ˮ����������Ӧ�ڼ�ʯ�Һ���������һ��װ�м�ʯ�ҵ�װ�á�
���㣺 ��ѧʵ��
������ ������ҪĿ���Dzⶨ������̼���������������������Ҳ�ܱ���ʯ�����գ����������ն�����̼����ǰӦ���������������������ȥ������������һ�������Ը��������Һ����ˮ������Ʒ����Һ����������������Ƿ������

 ���м����ʵ�鷽�����ⶨ����NH4HCO3��������������SO2������KMnO4�ķ�Ӧԭ����SO2��MnO4����H��-->SO42����Mn2����H2O ��
���м����ʵ�鷽�����ⶨ����NH4HCO3��������������SO2������KMnO4�ķ�Ӧԭ����SO2��MnO4����H��-->SO42����Mn2����H2O ��
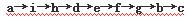 ���ش��������⣺
���ش��������⣺