��Ŀ����
��Ӳ�����ϣ���90%����2.5%þ��7.5%ͭ��������ȡ����KAl��SO4��2��12H2O��ij̽��С�����������ʵ�飮

��ش��������⣺
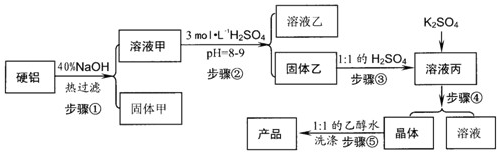
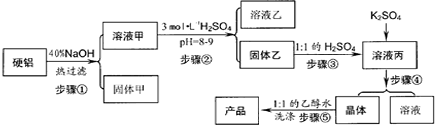
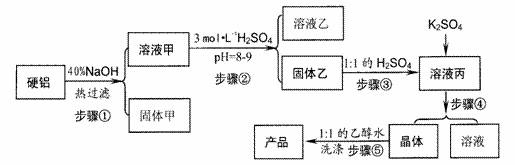
��1��д������۵����ӷ���ʽ
��2������ܰ����������ڣ��ֱ���
��3������ݲ�ֱ����ˮϴ��ԭ����
��ij����С��ͬѧ����Һ̬�̺�������ʵ��������£�
ԭ����Һ̬��
��NH4��2SO4��Һ
NH3
��NH4��2B4O7��Һ���ñ�����ζ�
���裺
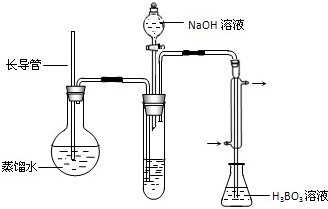
�����ձ��м���10.00mLҺ̬�̺����Լ������ȳ�ַ�Ӧ��
�ڽ���ӦҺת�Ƶ����Թ��У�
�۰�����װ����ˮ������NH3����������H3BO3��Һ���գ�����װ��δ��������

��ȡ����ƿ���μ�ָʾ������0.1000mol?L-1�����Һ�ζ���
���ظ��ⶨ���Σ�����10.00mL����ˮ����Һ̬�̽�������������
���ݼ�¼���£�
�ش��������⣺
��1���ζ�ʱ��NH4��2B4O7����ת��ΪH3BO3����Ӧ�Ļ�ѧ����ʽΪ
��2������۵�ʵ��װ������Ҫ���ȵ�������
��3�����4�ſհ���ʵ���Ŀ����
��4�������Һ̬�̵ĺ�����Ϊ

��ش��������⣺
��1��д������۵����ӷ���ʽ
Al��OH��3+3H+=Al3++3H2O
Al��OH��3+3H+=Al3++3H2O
����2������ܰ����������ڣ��ֱ���
����
����
����ȴ�ᾧ������
����
����3������ݲ�ֱ����ˮϴ��ԭ����
���ٲ�Ʒ����ʧ
���ٲ�Ʒ����ʧ
����ij����С��ͬѧ����Һ̬�̺�������ʵ��������£�
ԭ����Һ̬��
| ||
| ���� |
| ||
| ���� |
| ||
| ���� |
���裺
�����ձ��м���10.00mLҺ̬�̺����Լ������ȳ�ַ�Ӧ��
�ڽ���ӦҺת�Ƶ����Թ��У�
�۰�����װ����ˮ������NH3����������H3BO3��Һ���գ�����װ��δ��������

��ȡ����ƿ���μ�ָʾ������0.1000mol?L-1�����Һ�ζ���
���ظ��ⶨ���Σ�����10.00mL����ˮ����Һ̬�̽�������������
���ݼ�¼���£�
| ʵ���� | ��Ʒ�����Լ� | �������� �����mL�� |
| 1 | 10.00mLҺ̬�̡�0.2g������20mLŨ���� | 33.45 |
| 2 | 10.00mLҺ̬�̡�0.2g������20mLŨ���� | 33.55 |
| 3 | 10.00mLҺ̬�̡�0.2g������20mLŨ���� | 33.50 |
| 4 | 10.00mL����ˮ��0.2g������20mLŨ���� | 1.50 |
��1���ζ�ʱ��NH4��2B4O7����ת��ΪH3BO3����Ӧ�Ļ�ѧ����ʽΪ
��NH4��2B4O7+2HCl+5H2O=4H3BO3+2NH4Cl
��NH4��2B4O7+2HCl+5H2O=4H3BO3+2NH4Cl
����2������۵�ʵ��װ������Ҫ���ȵ�������
������
������
�����������ƣ��������ܵ���������ȫ��
��ȫ��
����3�����4�ſհ���ʵ���Ŀ����
���������Լ���ʵ�������������������
���������Լ���ʵ�������������������
����4�������Һ̬�̵ĺ�����Ϊ
4.48
4.48
mg?mL-1����������1��Ӳ���������������Ʒ�Ӧ����ƫ�����ƣ�����Һ����ƫ�����ƣ�����Һ���м������ᣬ����Һ����pHֵ��ƫ������������ӷ�Ӧ�����������������������Թ�����Ϊ����������������Ҽ������ᣬ�������������ᷴӦ������������ˮ��
��2������Һ�����������������м�������أ��û����Һ��ͨ���������ᾧ�������Ƶ�������
��3�������ھƾ��е��ܽ��С���þƾ�ϴ�Ӽ��ٲ�Ʒ����ʧ��
��1���μ����ᣨNH4��2B4O7ת��ΪH3BO3����Ļ��ϼ�δ�仯���������Ƹ��ֽⷴӦ�������Ȼ�����ɣ�
��2����ˮ������NH3���������Լ���Բ����ƿ����ˮ�����������Թ��н����ɵİ���������
���Ȳ���ˮ������װ����ѹǿ���ӣ���ֹװ����ѹ�����������Σ�գ���ȴʱ��ֹ������������ȫ�����ã�
��3��ʵ�������������ر�����Ӱ������ȵģ�����ͨ���հ���ʵ�飬�Ա�ʵ��Ľ�����ų��ر�����Ӱ�죬����϶�Ϊ������ʵ�������Ч����ʹʵ��������˵������
��4������NԪ���غ㣬���ζ���Ӧ�ҳ�Nԭ����HCl�Ĺ�ϵʽ�����ݹ�ϵʽ���㣬ע����������ȡ3�εζ���ƽ��ֵ��ȥ1.5ml��������ʱ������������Ϊ1.5ml����
��2������Һ�����������������м�������أ��û����Һ��ͨ���������ᾧ�������Ƶ�������
��3�������ھƾ��е��ܽ��С���þƾ�ϴ�Ӽ��ٲ�Ʒ����ʧ��
��1���μ����ᣨNH4��2B4O7ת��ΪH3BO3����Ļ��ϼ�δ�仯���������Ƹ��ֽⷴӦ�������Ȼ�����ɣ�
��2����ˮ������NH3���������Լ���Բ����ƿ����ˮ�����������Թ��н����ɵİ���������
���Ȳ���ˮ������װ����ѹǿ���ӣ���ֹװ����ѹ�����������Σ�գ���ȴʱ��ֹ������������ȫ�����ã�
��3��ʵ�������������ر�����Ӱ������ȵģ�����ͨ���հ���ʵ�飬�Ա�ʵ��Ľ�����ų��ر�����Ӱ�죬����϶�Ϊ������ʵ�������Ч����ʹʵ��������˵������
��4������NԪ���غ㣬���ζ���Ӧ�ҳ�Nԭ����HCl�Ĺ�ϵʽ�����ݹ�ϵʽ���㣬ע����������ȡ3�εζ���ƽ��ֵ��ȥ1.5ml��������ʱ������������Ϊ1.5ml����
����⣺��1���������������ᷴӦ������������ˮ�����ӷ���ʽΪAl��OH��3+3H+=Al3++3H2O��
�ʴ�Ϊ��Al��OH��3+3H+=Al3++3H2O��
��2������������Һ�м�������أ��û����Һ��ͨ���������ᾧ�������Ƶ��������ʴ�Ϊ�����������ˣ�
��3�������ھƾ��е��ܽ��С���þƾ�ϴ�Ӽ��ٲ�Ʒ����ʧ���ʴ�Ϊ�����ٲ�Ʒ����ʧ��
��1����Ļ��ϼ�δ�仯���������Ƹ��ֽⷴӦ����Ӧ����ʽΪ��NH4��2B4O7+2HCl+5H2O=4 H3BO3+2NH4Cl��
�ʴ�Ϊ����NH4��2B4O7+2HCl+5H2O=4 H3BO3+2NH4Cl��
��2������Բ����ƿ����ˮ�����������Թ��н����ɵİ������������Ȳ���ˮ������װ����ѹǿ���ӣ������ܷ�ֹװ����ѹ�����������Σ�գ���ȴʱ��ֹ������������ȫ�����ã�
�ʴ�Ϊ��Բ����ƿ����ȫ�ܣ�
��3������ʹ��Ŀ�������������Լ���ʵ�������������������ʴ�Ϊ�����������Լ���ʵ������������������
��4����������Ϊ
-1.5ml=32.00ml=0.03200L��
��10ml��Һ̬�̵ĺ�����������Ϊmg����
2N����NH4��2B4O7��2HCl
28g 2mol
mg 0.03200L��0.1000mol/L
����m=28g��
=0.04480g��
��Һ̬�̵ĺ�����Ϊ
=4.48mg/ml��
�ʴ�Ϊ��4.48��
�ʴ�Ϊ��Al��OH��3+3H+=Al3++3H2O��
��2������������Һ�м�������أ��û����Һ��ͨ���������ᾧ�������Ƶ��������ʴ�Ϊ�����������ˣ�
��3�������ھƾ��е��ܽ��С���þƾ�ϴ�Ӽ��ٲ�Ʒ����ʧ���ʴ�Ϊ�����ٲ�Ʒ����ʧ��
��1����Ļ��ϼ�δ�仯���������Ƹ��ֽⷴӦ����Ӧ����ʽΪ��NH4��2B4O7+2HCl+5H2O=4 H3BO3+2NH4Cl��
�ʴ�Ϊ����NH4��2B4O7+2HCl+5H2O=4 H3BO3+2NH4Cl��
��2������Բ����ƿ����ˮ�����������Թ��н����ɵİ������������Ȳ���ˮ������װ����ѹǿ���ӣ������ܷ�ֹװ����ѹ�����������Σ�գ���ȴʱ��ֹ������������ȫ�����ã�
�ʴ�Ϊ��Բ����ƿ����ȫ�ܣ�
��3������ʹ��Ŀ�������������Լ���ʵ�������������������ʴ�Ϊ�����������Լ���ʵ������������������
��4����������Ϊ
| 33.45ml+33.55ml+33.50ml |
| 3 |
��10ml��Һ̬�̵ĺ�����������Ϊmg����
2N����NH4��2B4O7��2HCl
28g 2mol
mg 0.03200L��0.1000mol/L
����m=28g��
| 0.03200ml��0.1000mol/L |
| 2mol |
��Һ̬�̵ĺ�����Ϊ
| 44.8mg |
| 10ml |
�ʴ�Ϊ��4.48��
����������֪ʶ�漰Ԫ�ػ����ʵ��ԭ������ѧ����ȣ����ؿ���ѧ����ʵ�鷽�����⡢Ԫ�ػ������֪ʶ���ѶȽϴ��״���Ϊ����Һ̬�̵ĺ�����ʱ�������ӦΪ3�εζ���ƽ��ֵ��ȥ1.5ml��������ʱ������������Ϊ1.5ml����

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
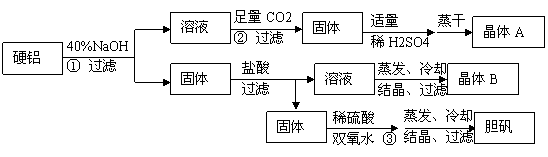 (8��)����Ӳ�����ϣ���90%����2.5%þ��7.5%ͭ�������ã�ij̽��С�����������ʵ�顣
(8��)����Ӳ�����ϣ���90%����2.5%þ��7.5%ͭ�������ã�ij̽��С�����������ʵ�顣