��Ŀ����
����Ŀ������Ԫ���ڻ�ѧ���к���Ҫ�ĵ�λ�������仯����㷺Ӧ�������Ų��ϡ��������ϡ���ȼ�ϲ��ϡ����ϲ��ϵȸ��²������ش��������⡣
��1��������һ���ܵ��߶ȹ�ע����ĥͿ�ϣ��������������ı��汣���㡣��ͼ��������ľ���ʾ��ͼ��������Ļ�ѧʽΪ_____���þ���ľ���������_______��
��2������(H3BO3)��һ��Ƭ��״�ṹ��ɫ���壬���ڵ�H3BO3���Ӽ�ͨ���������[����ͼ]��
�����������B�������_______�����ӣ�1molH3BO3�ľ�������_______mol�����
����������ˮ�����������һˮ������B(OH)3��H2O����������������[B(OH)4]����H�����ӡ�������Ϊ____________Ԫ�ᣬ[B(OH)4]�����еĻ�ѧ������Ϊ________��
��3����(�������)�ٷ��ӽṹ��ͼ:
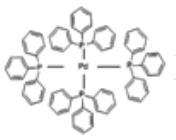
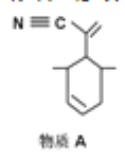
Pԭ���������������̬Χ������ԭ�������ϣ���ԭ�ӵ��ӻ��������Ϊ_____���жϸ�������ˮ���ܽ�Ȳ����Խ���________�������ʿ�������ͼ��ʾ����A�ĺ��ɡ�����A��̼ԭ���ӻ����������_______����һ��A����������̼ԭ����ĿΪ_____��
��4����ͼʾ�б�ʾ���ģ�������좣��ٷ�������λ��____________
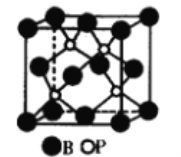
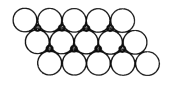
��5��NiO����ṹ��NaCl�������ƣ��侧�����ⳤΪacm����þ����о����������������Ӻ˼�ľ���Ϊ_______(�ú���a�Ĵ���ʽ��ʾ)����һ���¶��£�Ni0��������Է��ط�ɢ���γ��������Ӳ�������ͼ����������Ϊ�����������µ������У�������������У���ʽ������ÿƽ��������Ϸ�ɢ�ĸþ��������Ϊ_______g�������ӵİ뾶Ϊ1.40��10-10m����![]() ��l.7����
��l.7����
���𰸡� BP ԭ�Ӿ��� 3 3 һ ���ۼ�(���Լ�)����λ�� sp3 ������ˮ��ˮΪ���Է��ӣ���(�������)�ٷ���Ϊ�Ǽ��Է��ӣ����Ӽ��Բ����ƣ��ʲ����� sp��sp2��sp3 3 
![]() 1.84��10-3
1.84��10-3
����������1����ͼ����֪��,Bԭ�ӷֱ�������Pԭ���γɹ��ۼ�,������Ļ�ѧʽΪBP,������ڵ�������Ϊ���ۼ�,���������Թ��ۼ��γɵĿռ���״�ṹ�ľ�������ԭ�Ӿ���;��ˣ�������ȷ����:BP;ԭ�Ӿ�����
(2) ��Bԭ���������3������,ÿ��Bԭ���γ�3�����ۼ�,�������������B�������6������;һ��H3BO3���Ӷ�Ӧ��6�����,һ�������Ӧ��2��H3BO3����,��˺���H3BO3���ӵľ�������3���,��ˣ�������ȷ����:3;3;
����������ˮ�����������һˮ������![]() ,��������������
,��������������![]() ����,�������ܵ����һ����ԭ��,������������һԪ��;
����,�������ܵ����һ����ԭ��,������������һԪ��; ![]() ���еĻ�ѧ������Ϊ���ۼ�����λ��,��ˣ�������ȷ����:һ;���ۼ�����λ����
���еĻ�ѧ������Ϊ���ۼ�����λ��,��ˣ�������ȷ����:һ;���ۼ�����λ����
��3��Pԭ���������������̬Χ������ԭ��������,��ԭ�ӵ��ӻ��������Ϊsp3��ˮΪ���Է���,��(�������)�ٷ���Ϊ�Ǽ��Է���,���Ӽ��Բ�����,�ʲ�������
A������Cԭ�Ӿ�û�й¶Ե���,������Cԭ���ӻ������ĿΪ2,˫����̼ԭ���ӻ������ĿΪ3,����̼ԭ���ӻ������ĿΪ4,̼ԭ���ӻ���ʽΪ:SP��SP2��SP3������4����ͬ��ԭ�ӻ�ԭ���ŵ�̼ԭ��Ϊ����̼ԭ��,��������3������̼ԭ����
��4��)Pd���пչ��,Pԭ����1�Թ¶Ե���,�ṩ�¶Ե�����Pd�γ���λ��,��������λ��Ϊ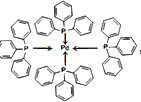
��5�������Ȼ��ƵĽṹ֪�������Ӻ����ڵ�������֮��ľ���Ϊ1/2a��������������������Ӻ˼�ľ����Ǿ�������������Ӻ������Ӿ����![]() ���������������
���������������![]() acm
acm
����ͼƬ֪��ÿ����������ռ�����=1.40��10-10m��1.40��10-10m��sin60�㣬��ÿƽ�����е�����������=![]() ��ÿ��������������=
��ÿ��������������=![]() g������ÿƽ�����е�����������=
g������ÿƽ�����е�����������=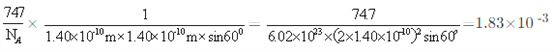 .��
.��