��Ŀ����
�£�N2H4���㷺���ڻ���ƽ������л��ϳɼ����ȼ�ϡ���ش���������
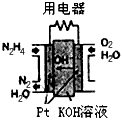
��1��ֱ����ȼ�ϵ��ԭ����ͼ��ʾ��ͨ��N2H4�ļ�Ϊ��ص�__________���������������
��1��ֱ����ȼ�ϵ��ԭ����ͼ��ʾ��ͨ��N2H4�ļ�Ϊ��ص�__________���������������
��2���������N2O4��������������ȼ�ϣ���֪��
N2(g)+2O2(g)=2NO2(g) ��H=-67.7kJ��mol-1��
N2H4��g��+O2��g��=N2(g)+2H2O(g) ��H=-534.0kJ��mol-1
2NO2��g�� N2O4(g) ��H=-52.7kJ��mol-1
N2O4(g) ��H=-52.7kJ��mol-1
��д����̬������̬������������ȼ�����ɵ�������̬ˮ���Ȼ�ѧ����ʽ��_____________________��
��3���µ�ˮ���ˮ���£�N2H4��2H2O����һ��ǿ��ԭ����ij���ױ�����Fe-Al���ϴ�����ˮ���¿�ѡ���Ի�ԭ�����������Ʊ����ȱ�����ij��ʵ�鲿�ּ�¼����
N2(g)+2O2(g)=2NO2(g) ��H=-67.7kJ��mol-1��
N2H4��g��+O2��g��=N2(g)+2H2O(g) ��H=-534.0kJ��mol-1
2NO2��g��
 N2O4(g) ��H=-52.7kJ��mol-1
N2O4(g) ��H=-52.7kJ��mol-1 ��д����̬������̬������������ȼ�����ɵ�������̬ˮ���Ȼ�ѧ����ʽ��_____________________��
��3���µ�ˮ���ˮ���£�N2H4��2H2O����һ��ǿ��ԭ����ij���ױ�����Fe-Al���ϴ�����ˮ���¿�ѡ���Ի�ԭ�����������Ʊ����ȱ�����ij��ʵ�鲿�ּ�¼����
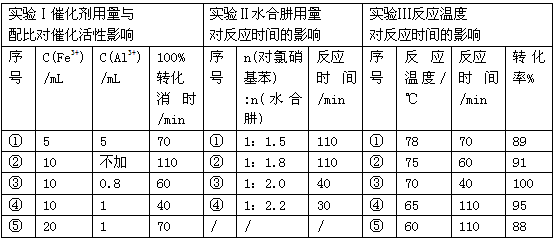
��ֱ��ʵ�����ϳ������������Ϊ_________________��ѡ����Ţ٢ڢۢܢݣ���
��4����֪��2NO2��g��==N2O4��g�� ��H=-57.20kJ��mol-1��һ���¶��£����ܱ������з�Ӧ
2NO2��g�� N2O4��g���ﵽƽ�⡣������������ʱ�����д�ʩ�����NO2ת���ʵ���__________������ĸ��
N2O4��g���ﵽƽ�⡣������������ʱ�����д�ʩ�����NO2ת���ʵ���__________������ĸ��
A����СNO2��Ũ�� B�������¶� C������NO2��Ũ�� D�������¶�
��5��17�桢1.01��105Pa���ܱ�������N2O4��NO2�Ļ������ﵽƽ��ʱ��c��NO2��=0.0300mol��L-1��
c��N2O4��=0.0120 mol��L-1�����㷴Ӧ2NO2��g�� N2O4��g����ƽ�ⳣ��K��
N2O4��g����ƽ�ⳣ��K��
_______________________________
��4����֪��2NO2��g��==N2O4��g�� ��H=-57.20kJ��mol-1��һ���¶��£����ܱ������з�Ӧ
2NO2��g��
 N2O4��g���ﵽƽ�⡣������������ʱ�����д�ʩ�����NO2ת���ʵ���__________������ĸ��
N2O4��g���ﵽƽ�⡣������������ʱ�����д�ʩ�����NO2ת���ʵ���__________������ĸ��A����СNO2��Ũ�� B�������¶� C������NO2��Ũ�� D�������¶�
��5��17�桢1.01��105Pa���ܱ�������N2O4��NO2�Ļ������ﵽƽ��ʱ��c��NO2��=0.0300mol��L-1��
c��N2O4��=0.0120 mol��L-1�����㷴Ӧ2NO2��g��
 N2O4��g����ƽ�ⳣ��K��
N2O4��g����ƽ�ⳣ��K�� _______________________________
��1������
��2��2N2H4��g��+N2O4��g��=3N2��g��+4H2O��g����H=-947 .6 kJ�� mol-1
��3���ܢܢ�
��4��BC
��5��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
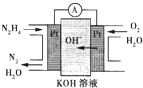 �£�N2H4���ֳ��������㷺���ڻ���ƽ������л��ϳɼ�ȼ�ϵ�أ�NO2�Ķ�����N2O4���ǻ���г��õ�����������ش��������⣺
�£�N2H4���ֳ��������㷺���ڻ���ƽ������л��ϳɼ�ȼ�ϵ�أ�NO2�Ķ�����N2O4���ǻ���г��õ�����������ش��������⣺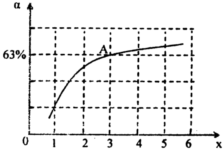 ��2013?������һģ���£�N2H4���Ͱ��ǵ������ֳ���������ڿ�ѧ�������������й㷺Ӧ�ã��밴Ҫ��ش��������⣺
��2013?������һģ���£�N2H4���Ͱ��ǵ������ֳ���������ڿ�ѧ�������������й㷺Ӧ�ã��밴Ҫ��ش��������⣺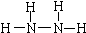
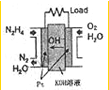 �£�N2H4���㷺���ڻ���ƽ������л��ϳɼ����ȼ�ϣ��Ȼش���������
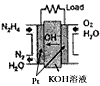
�£�N2H4���㷺���ڻ���ƽ������л��ϳɼ����ȼ�ϣ��Ȼش���������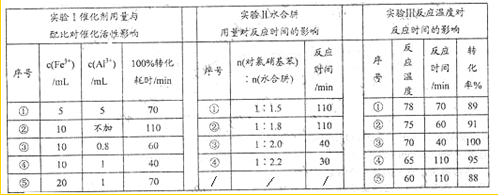
 N2O4(g) ��H=-52��7kJ��mol-1 ��д����̬������̬������������ȼ�����ɵ�������̬ˮ���Ȼ�ѧ����ʽ��__________________________��
N2O4(g) ��H=-52��7kJ��mol-1 ��д����̬������̬������������ȼ�����ɵ�������̬ˮ���Ȼ�ѧ����ʽ��__________________________�� 