��Ŀ����
����Ŀ���밴����Ҫ����գ�
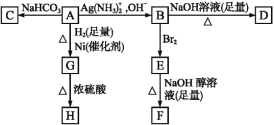
(1)���ĵ���ʽ__________��
(2)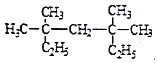 ϵͳ������Ϊ_________________��
ϵͳ������Ϊ_________________��
(3)����ʽ![]() ��ʾ���л���Ľṹ��ʽ��____________��
��ʾ���л���Ľṹ��ʽ��____________��
(4)����A��ͬ��ͬѹ���������ܶ���H2��36��������A�Ľṹ��ʽΪ________________��
(5)��Է�������Ϊ70��ϩ���ķ���ʽΪ_________������ϩ��������H2�ӳɺ������ɺ�3���������������ϩ�����ܵĽṹ��ʽΪ_________��
(6)д�����з�Ӧ�Ļ�ѧ����ʽ��
��ʵ������ȡ��Ȳ_____________________________________________________��
��2 -�ȱ������ȥ��Ӧ_____________________________________________________��
���üױ���TNT�ķ�Ӧ_____________________________________________________��
���𰸡�![]() 3��3��5��5-�ļ�����
3��3��5��5-�ļ����� ![]() CH3CH2CH2CH2CH3��
CH3CH2CH2CH2CH3��![]() ��
��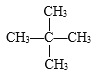 C5H10
C5H10 ![]() ��
��![]() ����CH3��2CHCH=CH2 CaC2+2H2O��Ca(OH)2+C2H2�� CH3CHClCH3+NaOH
����CH3��2CHCH=CH2 CaC2+2H2O��Ca(OH)2+C2H2�� CH3CHClCH3+NaOH![]() CH2=CH-CH3+NaCl+H2O
CH2=CH-CH3+NaCl+H2O ![]() +3HNO3
+3HNO3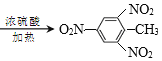 +3H2O
+3H2O
��������
(1)Cԭ�Ӽ۵���Ϊ4��Cԭ�ӵ�3��������3��Hԭ���γ�3�Թ��õ��Ӷԣ�
(2)����������ϵͳ������������
(3)���ݽṹ��ʽ�ͼ���ʽ����дԭ����д��
(4)�ȸ�������ܶȼ�����������Է���������Ȼ����������ķ���ʽͨʽ��������к��е�C��Hԭ����Ŀ���õ�����ʽ��
(5)����ϩ���ķ���ʽͨʽ��CnH2n�������Է����������������C��Hԭ�Ӹ������õ���ϩ���ķ���ʽ��Ȼ���ϸ�ϩ��������H2�ӳɺ����������Ľṹ�ص㣬�õ���ϩ�����ܵĽṹ��ʽ��
(6)�ٵ�ʯ��ˮ��Ӧ������Ȳ���������ƣ�
��2 -�ȱ�����NaOH���Ҵ���Һ�ڼ���ʱ����ȥ��Ӧ��
�ۼױ���Ũ�����ڼ���ʱ����ȡ����Ӧ����TNT��ˮ��
(1)Cԭ�Ӽ۵���Ϊ4��Cԭ�ӵ�3��������3��Hԭ���γ�3�Թ��õ��Ӷԣ���˼��ĵ���ʽΪ��![]() ��
��
(2)ѡ��Cԭ��������̼��Ϊ����������֧���Ͻ���һ�˸������ϵ�Cԭ�ӱ�ţ���ȷ��֧����λ�ã���֧����ȡ�������û����������Ϊ3��3��5��5-�ļ����飻
(3)�û����������Ϊ2-�һ�-1��3-����ϩ����ṹ��ʽ��![]() ��
��
(4)����A��ͬ��ͬѹ���������ܶ���H2��36���������������Է���������M=2��36=72�����������ķ���ͨʽΪCnH2n+2��14n+2=72�����n=5��������A�Ľṹ��ʽΪC5H12�����ܵĽṹ��ʽΪCH3CH2CH2CH2CH3��![]() ��
�� ��
��
(5)ϩ���ķ���ͨʽΪCnH2n����������Է�������Ϊ70����14n=70�����n=5���ʸ�ϩ���ķ���ʽΪC5H10������ϩ��������H2�ӳɺ������ɺ�3������������˵����ϩ���ӳɲ���Ϊ��CH3��2CHCH2CH3�����ϩ�����ܵĽṹ��ʽΪ![]() ��
��![]() ����CH3��2CHCH=CH2��
����CH3��2CHCH=CH2��
(6)�ٵ�ʯ��ˮ��Ӧ������Ȳ���������ƣ���Ӧ�Ļ�ѧ����ʽΪCaC2+2H2O��Ca(OH)2+C2H2����
��2 -�ȱ�����NaOH���Ҵ���Һ�ڼ���ʱ����ȥ��Ӧ����Ӧ�Ļ�ѧ����ʽΪCH3CHClCH3+NaOH![]() CH2=CH-CH3+NaCl+H2O��
CH2=CH-CH3+NaCl+H2O��
�ۼױ���Ũ�����ڼ���ʱ����ȡ����Ӧ����TNT��ˮ����Ӧ�Ļ�ѧ����ʽΪ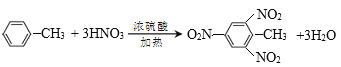 ��
��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ����84����Һ����1984�걱��ijҽԺ����ʹ�ö����������ճ�������ʹ�ù㷺������Ч�ɷ���NaClO��ij��ѧ�о���ѧϰС����ʵ�����Ʊ�NaClO��Һ������������̽���ͳɷֲⶨ
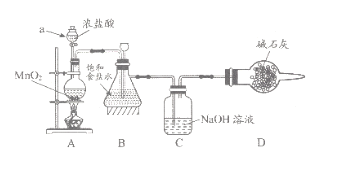
��1����ѧϰС�鰴��ͼװ�ý���ʵ��(���ּг�װ��ʡȥ)����Ӧһ��ʱ��ֱ�ȡB��Cƿ�е���Һ����ʵ�飬ʵ���������±���
��֪��1������NaClO��ҺpHΪ11��
2��25��Cʱ��������볣��Ϊ��H2CO3��K1=4.4��10��7��K2=4.7��10��11��HClO��K=3��10��8
Bƿ | Cƿ | |
ʵ��1��ȡ�����μ���ɫʯ����Һ | ��죬����ɫ | ����������ɫ |
ʵ��2���ⶨ��Һ��pH | 3 | 12 |
�ش��������⣺
������a������_____��װ��A�з�����Ӧ�����ӷ���ʽ________��
��ʵ��1��Bƿ��Һ�в��������ԭ����_________��
������Cƿ��Һ���� NaHCO3��Һ�������������������ʵ�飬Cƿ����Ϊ��ʵ��1����ɫʯ����Һ������ɫ��ʵ��2����Һ��pH=7�����ƽ���ƶ�ԭ��������ɫʯ����Һ������ɫ��ԭ��______��
��2���ⶨCƿ��Һ��NaClO����(��λ��g/L)��ʵ�鲽�����£�
��ȡCƿ��Һ20ml����ƿ�У����������ữ���������KI��Һ���ǽ�ƿ�����ڰ�����ַ�Ӧ��
����0.1000mol/LNa2S2O3����Һ�ζ���ƿ�е���Һ��ָʾ����ʾ�յ���ظ�����2~3�Σ�Na2S2O3��Һ��ƽ������Ϊ24.00ml��(��֪��I2+2S2O32��=2I��+S4O62��)
�ٲ�����Cƿ�з�����Ӧ�����ӷ���ʽΪ_______��
�ڲ����ͨ��ѡ��___��ָʾ�����ζ����յ������______��
��Cƿ��Һ��NaClO����Ϊ_____g/L(����2λС��)
����Ŀ����һ���¶��£�10 mL 0.40 mol/L H2O2��Һ�������ֽ⣬��ͬʱ�̲������O2�����(������Ϊ��״��)���±���������Һ����仯��������������ȷ����
t/min | 0 | 2 | 4 | 6 | 8 | 10 |
V(O2)/mL | 0.0 | 9.6 | 16.8 | 22.4 | 26.5 | 29.9 |
A.�÷�Ӧ����MnO2��FeCl3��Һ��Ϊ����
B.0��4 min��ƽ����Ӧ����v(H2O2)��3.75��10-2mol/(L��min)
C.0��10 min����������ķֽ������ӿ�
D.��Ӧ��6 minʱ��H2O2�ֽ���50%