��Ŀ����
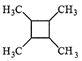
����Ŀ���屽����ɫ��״Һ�壬���б�����ζ����������Ҫ�����ܼ��������Լ����л��ϳɵȡ�ij��ѧС����������ͼ��ʾ��װ��ͨ��ʵ��̽����
��.����Һ�巢��ȡ����Ӧ����֪��MnO2��2NaBr��2H2SO4![]() Br2����MnSO4��Na2SO4��2H2O
Br2����MnSO4��Na2SO4��2H2O
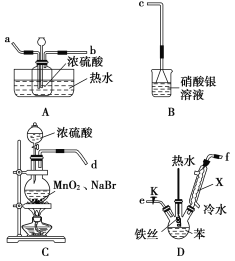
(1)����X������______________ ��ʵ�����Ʊ��屽�Ļ�ѧ����ʽΪ____________________��
������ȡ���屽�л��ܽ������Br2 ��������________ϴ�ӳ�Br2��
A��Na2SO3��Һ B��H2O C���Ҵ���Һ D��NaOH��Һ
(2)��ȡ�屽��ʵ������������£�
������װ�ã���ӿ�˳��Ϊ(����ĸ)��d��________��________��________��________��c��
�ڼ��װ�õ������ԣ�
��C�м������ҩƷ���μ�Ũ���ᣬ�ٴ�D������K����ȼC���ƾ��ƣ���Dװ���е���˿������Һ�У�һ��ʱ���D��Һ����ڣ�ԭ����_____________________��
�ܹر�C�з�Һ©����������
(3)A�жԹ��ƿ������ˮԡ��Ŀ��_________________________________��
(4)��B���е���ɫ�������ɣ��ܷ�ȷ������Һ�巢����ȡ����Ӧ��________(����������������)�������е�ԭ��_________________________________��
��.̽������Һ���ڴ��������µķ�Ӧ������
�������ף���Br����ǿ�����ԣ����Ժ�ˮ���ӷ���������ԭ��Ӧ���ڱ���Һ�����廯�����µķ�Ӧ��������������(��д���пհ�)
��һ����Br2��FeBr3![]() Br����FeBr
Br����FeBr![]() �ڶ�����
�ڶ�����![]()
����ɵ�������Ӧ��__________________________����С�齫Aװ����Ũ�����滻��ϡ����ʱʵ��ʧ�ܣ��Դӷ�Ӧ�����Ʋ���ܵ�ԭ����___________________________________��
���𰸡����������� ![]() ��Br2
��Br2![]()
![]() ��HBr AD a b e f �÷�Ӧ�Ƿ��ȷ�Ӧ ��ֹ���������� �� D�л�ӷ���������Br2��Br2Ҳ���Ժ���������������ɫ���� FeBr4-��
��HBr AD a b e f �÷�Ӧ�Ƿ��ȷ�Ӧ ��ֹ���������� �� D�л�ӷ���������Br2��Br2Ҳ���Ժ���������������ɫ���� FeBr4-��![]() ��FeBr3��
��FeBr3��![]() ��HBr ��ˮ������£�������Br+
��HBr ��ˮ������£�������Br+
��������
cװ�÷�����Ӧ��MnO2+2NaBr+2H2SO4 ![]() Br2��+MnSO4+Na2SO4+2H2O���Ʊ��嵥�ʣ�Ϊ��ֹ��������������A�жԹ��ƿ������ˮԡ����������D���뱽������Ӧ��
Br2��+MnSO4+Na2SO4+2H2O���Ʊ��嵥�ʣ�Ϊ��ֹ��������������A�жԹ��ƿ������ˮԡ����������D���뱽������Ӧ��![]() �������������������δ��Ӧ���������廯�⣬��ֹ��Ⱦ�������ݴ˷�������
�������������������δ��Ӧ���������廯�⣬��ֹ��Ⱦ�������ݴ˷�������
(1)����XΪ���������ܣ�ʵ�����ñ������ڴ����������·�Ӧ�Ʊ��屽����ѧ����ʽΪ��![]() ��
��
A��Br2������ǿ��������Na2SO3��Һ������������ˮ���Σ������ڷ�Һ���룬��A��ȷ��
B��Br2�������屽����������ˮ����ͨ����Һ���з��룬��B����
C���Ҵ���ˮ���ܣ��������õ��л��ܼ������ֲ㣬���ɷ�Һ���룬��C����
D��Br2������NaOH��Һ������������ˮ���Σ������ڷ�Һ���룬��D��ȷ��
��ΪAD��
(2)����������C�Ʊ�����d��a������ˮԡ��ֹ��������c��e����D�з�Ӧ����B������β������˳��Ϊd��a��b��e��f��c��
��C�м������ҩƷ��C���μ�Ũ���ᣬ�ٴ�D������K����ȼC���ƾ��ƣ��Ƶ���������Ӧ���÷�Ӧ�Ƿ��ȷ�Ӧ��������˿�ڻ��Һ�У�һ��ʱ���D��Һ����ڣ�
(3)Ϊ��ֹBr2����������A�жԹ��ƿ������ˮԡ��
(4)Br2�лӷ��ԣ�D�л�ӷ���������Br2��Br2Ҳ���Ժ���������������ɫ�������ʲ���ȷ������Һ�巢����ȡ����Ӧ��
��������ӦΪ�屽�����ɣ���ѧ��ӦΪ��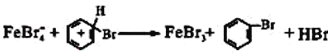 ����Aװ����Ũ�����滻��ϡ����ʱʵ��ʧ�ܣ���ˮ����£�������Br+��
����Aװ����Ũ�����滻��ϡ����ʱʵ��ʧ�ܣ���ˮ����£�������Br+��

����Ŀ��ʵ������ijЩ�������ȡ���ռ���β������װ����ͼ��ʾ��ʡ�Լгֺ;���װ�ã������ô�װ�úͱ����ṩ������������ʵ�飬�������ѡ����

ѡ�� | a�е����� | b�е����� | c���ռ������� | d�е����� |
A | Ũ��ˮ | CaO | NH3 | H2O |
B | Ũ���� | Na2SO3 | SO2 | NaOH��Һ |
C | ϡ���� | Cu | NO2 | H2O |
D | Ũ���� | MnO2 | Cl2 | NaOH��Һ |