��Ŀ����
�о������仯�����������������������о��м�Ϊ��Ҫ�����塣
(1)��Ȼ���д����Ÿ��̵ֹ���Ӧ��д��������������ʱ�Ĺ̵���Ӧ�Ļ�ѧ����ʽ��__________________
(2)��֪��Ӧ��
��2H2(g)+O2(g) 2H2O(g) ��H=-483.6kj/mol
2H2O(g) ��H=-483.6kj/mol
��N2(g)+3H2(g) 2NH3(g) ��H=-92.4kJ/mol
2NH3(g) ��H=-92.4kJ/mol
��4NH3(g)+5O2(g) 4NO(g)+6H2O(g) ��H=-905kJ/mol
4NO(g)+6H2O(g) ��H=-905kJ/mol
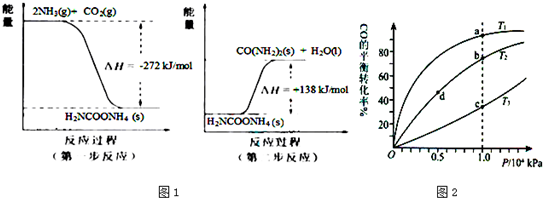
��Ӧ��4NH3(g)+3O2(g) 2N2(g)+6H2O(g)�ġ�H=___________����ͼ����һ���ܱ������ڣ��ڴ��������£�һ�����İ����������Ļ�������ڲ�ͬ�¶�ʱ�������P�����ﺬ�����¶ȵĹ�ϵͼ���¶Ƚϵ�ʱ�Է�Ӧ_______��ѡ��������֪��Ӧ��š��١��ڡ��ۻ�ܡ���Ϊ�������¶ȸ���900��ʱ��NO�����½���ԭ��____��
2N2(g)+6H2O(g)�ġ�H=___________����ͼ����һ���ܱ������ڣ��ڴ��������£�һ�����İ����������Ļ�������ڲ�ͬ�¶�ʱ�������P�����ﺬ�����¶ȵĹ�ϵͼ���¶Ƚϵ�ʱ�Է�Ӧ_______��ѡ��������֪��Ӧ��š��١��ڡ��ۻ�ܡ���Ϊ�������¶ȸ���900��ʱ��NO�����½���ԭ��____��
(1)��Ȼ���д����Ÿ��̵ֹ���Ӧ��д��������������ʱ�Ĺ̵���Ӧ�Ļ�ѧ����ʽ��__________________
(2)��֪��Ӧ��
��2H2(g)+O2(g)
 2H2O(g) ��H=-483.6kj/mol
2H2O(g) ��H=-483.6kj/mol ��N2(g)+3H2(g)
 2NH3(g) ��H=-92.4kJ/mol
2NH3(g) ��H=-92.4kJ/mol ��4NH3(g)+5O2(g)
 4NO(g)+6H2O(g) ��H=-905kJ/mol
4NO(g)+6H2O(g) ��H=-905kJ/mol ��Ӧ��4NH3(g)+3O2(g)
 2N2(g)+6H2O(g)�ġ�H=___________����ͼ����һ���ܱ������ڣ��ڴ��������£�һ�����İ����������Ļ�������ڲ�ͬ�¶�ʱ�������P�����ﺬ�����¶ȵĹ�ϵͼ���¶Ƚϵ�ʱ�Է�Ӧ_______��ѡ��������֪��Ӧ��š��١��ڡ��ۻ�ܡ���Ϊ�������¶ȸ���900��ʱ��NO�����½���ԭ��____��
2N2(g)+6H2O(g)�ġ�H=___________����ͼ����һ���ܱ������ڣ��ڴ��������£�һ�����İ����������Ļ�������ڲ�ͬ�¶�ʱ�������P�����ﺬ�����¶ȵĹ�ϵͼ���¶Ƚϵ�ʱ�Է�Ӧ_______��ѡ��������֪��Ӧ��š��١��ڡ��ۻ�ܡ���Ϊ�������¶ȸ���900��ʱ��NO�����½���ԭ��____�� 
(3)��ʵ�����У�ijͬѧ���������Լ���������ɵ�ʵ���ܹ�֤��NH3��H2O��������ʵ���_________��ѡ����ĸ���ţ���
a���ð�ˮ��������ʵ�飬���ݻ谵
b������ˮ����AlCl3��Һ�У�������ɫ����
c�������£���pH��ֽ���0.1mol/L��ˮ��pH<13
d����ʪ�����ɫʯ����ֽ���NH4Cl��ҺΪ��ɫ
(4)��һ���¶��£���һ�ݻ�Ϊ2L���ܱ������У�ͨ��һ����NO2��N2O4�Ļ�����壬������������ʵ�Ũ����ʱ��ı仯��ϵ��ͼ��ʾ��0��10min��N2O4�Ļ�ѧ��Ӧ����v(N2O4)=_______���ڵ�25minʱ���������������ʵ�Ũ��ͻȻ�����仯���������������ԭ����_________��35minʱ�� ��ӦN2O4 2NO2�Ļ�ѧƽ�ⳣ��K=________��
2NO2�Ļ�ѧƽ�ⳣ��K=________��
a���ð�ˮ��������ʵ�飬���ݻ谵
b������ˮ����AlCl3��Һ�У�������ɫ����
c�������£���pH��ֽ���0.1mol/L��ˮ��pH<13
d����ʪ�����ɫʯ����ֽ���NH4Cl��ҺΪ��ɫ
(4)��һ���¶��£���һ�ݻ�Ϊ2L���ܱ������У�ͨ��һ����NO2��N2O4�Ļ�����壬������������ʵ�Ũ����ʱ��ı仯��ϵ��ͼ��ʾ��0��10min��N2O4�Ļ�ѧ��Ӧ����v(N2O4)=_______���ڵ�25minʱ���������������ʵ�Ũ��ͻȻ�����仯���������������ԭ����_________��35minʱ�� ��ӦN2O4
 2NO2�Ļ�ѧƽ�ⳣ��K=________��
2NO2�Ļ�ѧƽ�ⳣ��K=________�� 
(1)N2(g)+O2(g)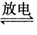 2NO(g)
2NO(g)
(2)-1266kJ/mol���ܣ�����NO�ķ�ӦΪ���ȷ�Ӧ���¶ȸ���900��ʱ�������¶ȣ�ƽ�������ƶ��������½�
(3)e
(4)0.02mol/(L��min)��������NO2��Ũ�ȣ�0.9mol/L
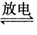 2NO(g)
2NO(g)(2)-1266kJ/mol���ܣ�����NO�ķ�ӦΪ���ȷ�Ӧ���¶ȸ���900��ʱ�������¶ȣ�ƽ�������ƶ��������½�
(3)e
(4)0.02mol/(L��min)��������NO2��Ũ�ȣ�0.9mol/L

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ