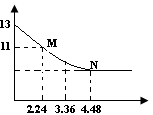
��Ŀ����
(8��)A��B������ͬ���ʣ�����H��N��O��Na�е���������Ԫ����ɵ�ǿ����ʣ�A��ˮ��Һ�ʼ��ԣ�B��ˮ��Һ�����ԣ����ҳ�A��B���ܵ�������ϣ�Ҫ����ͬŨ��ʱ��A1��Һ��ˮ�ĵ���̶�С��A2��Һ��ˮ�ĵ���̶ȣ���ͬŨ��ʱ��B1��Һ��ˮ�ĵ���̶�С��B2��Һ��ˮ�ĵ���̶ȡ�
��1��д����ѧʽA1________��A2________��B1________��B2________��
��2����ͬ�¶��£���A1��B1�����ʵ���Ũ�����ʱ������Һ��ˮ������������ӵ����ʵ���Ũ��֮��Ϊ_______________________ _��
��3���0.1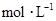 ��
�� ��Һ������Ũ�ȴ�С���� ________________��
��Һ������Ũ�ȴ�С���� ________________��
��4��B1��Һ��ˮ�ĵ���̶�С��B2��Һ��ˮ�ĵ���̶ȣ�ԭ���� ________________��
��5����B1��B2����Һ��pH��5��������Һ��ˮ������������ӵ����ʵ���Ũ��֮��Ϊ__________��
��1��д����ѧʽA1________��A2________��B1________��B2________��
��2����ͬ�¶��£���A1��B1�����ʵ���Ũ�����ʱ������Һ��ˮ������������ӵ����ʵ���Ũ��֮��Ϊ_______________________ _��
��3���0.1
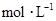 ��
�� ��Һ������Ũ�ȴ�С���� ________________��
��Һ������Ũ�ȴ�С���� ________________����4��B1��Һ��ˮ�ĵ���̶�С��B2��Һ��ˮ�ĵ���̶ȣ�ԭ���� ________________��
��5����B1��B2����Һ��pH��5��������Һ��ˮ������������ӵ����ʵ���Ũ��֮��Ϊ__________��
(1��NaOH��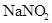 ��
��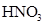 ��
��
��2��1��1
��3��
��4����������������������ˮ�ĵ��루1�֣���笠����ӵĴ��ڴٽ���ˮ�ĵ���
��5��1�� ��1��������1��
��1��������1�� ��
��
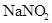 ��
��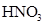 ��
��
��2��1��1
��3��

��4����������������������ˮ�ĵ��루1�֣���笠����ӵĴ��ڴٽ���ˮ�ĵ���
��5��1��
 ��1��������1��
��1��������1�� ��
����1��A1 ��Һ�е�ˮ�ĵ���̶�С��A2 ��Һ��ˮ�ĵ���̶ȣ�˵��A1�ǼA2����ˮ���Լ��ԡ�B1 ��Һ�е�ˮ�ĵ���̶�С��B2��Һ��ˮ�ĵ���̶ȣ�ͬ����֪B1���ᣬB2����ˮ�������ԡ�������ȷ�������A1 NaOH A2 NaNO2 B1 HNO3 B2 NH4NO3
(2)��Ũ�ȵ�NaOH ��HNO3��Һ��ǰ�ߵ�OH��Ũ������ߵ�H��Ũ����ȣ���ˮ�ĵ���ƽ�⣺H2O H����OH�������������൱��������Һ��ˮ������������ӵ����ʵ���Ũ��֮��Ϊ������
H����OH�������������൱��������Һ��ˮ������������ӵ����ʵ���Ũ��֮��Ϊ������
��3�� NaNO2��Һ�� ����ˮ�⣬��Һ�ʼ��ԣ���
����ˮ�⣬��Һ�ʼ��ԣ���
��4������������H������ˮ�ĵ��룬������淋������NH4+ˮ�⣬�ٽ�ˮ�ĵ���
��5��������Һ�е�OH��ȫ��������ˮ�ĵ��룬��1��10��9mol/L�����������Һ�е�H��ȫ��������ˮ�ĵ��룬��Ũ��Ϊ1��10��5mol/L��������Һ��ˮ������������ӵ����ʵ���Ũ��֮��Ϊ1��10��9��1��10��5=1��
(2)��Ũ�ȵ�NaOH ��HNO3��Һ��ǰ�ߵ�OH��Ũ������ߵ�H��Ũ����ȣ���ˮ�ĵ���ƽ�⣺H2O
 H����OH�������������൱��������Һ��ˮ������������ӵ����ʵ���Ũ��֮��Ϊ������
H����OH�������������൱��������Һ��ˮ������������ӵ����ʵ���Ũ��֮��Ϊ��������3�� NaNO2��Һ��
 ����ˮ�⣬��Һ�ʼ��ԣ���
����ˮ�⣬��Һ�ʼ��ԣ���
��4������������H������ˮ�ĵ��룬������淋������NH4+ˮ�⣬�ٽ�ˮ�ĵ���
��5��������Һ�е�OH��ȫ��������ˮ�ĵ��룬��1��10��9mol/L�����������Һ�е�H��ȫ��������ˮ�ĵ��룬��Ũ��Ϊ1��10��5mol/L��������Һ��ˮ������������ӵ����ʵ���Ũ��֮��Ϊ1��10��9��1��10��5=1��


��ϰ��ϵ�д�
�����Ŀ
 CO32-+H3O+
CO32-+H3O+