��Ŀ����
����Ŀ������ͼ���е����ߣ�������Ϊ���������������������Ϊ�������ʵ����������д������
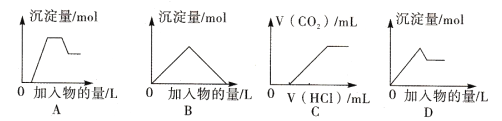
A. ͼA��ʾ��H+��Mg2+��Al3+��NH4+����Һ�еμ�NaOH��Һ�����������Ĺ�ϵ����
B. ͼB��ʾ�����ʯ��ˮ��ͨ�������ֱ̼�����������������Ĺ�ϵ����
C. ͼC��ʾ��NaOH��Na2CO3�Ļ��Һ�еμ��������CO2����Ĺ�ϵ����
D. ͼD��ʾ��������Һ�еμ�Ba��OH��2��Һ�����������Ĺ�ϵ����
���𰸡�C
�����������������A��H+��Mg2+��Al3+��NH4+����Һ��H+������OH-��Ӧ�����Կ�ʼ���������ΪAl3+����Ӧ������ˮ���������������ֳ�����Mg2+��OH-��Ӧ����������þ��������������������μӣ�NH4+��OH-��Ӧ����һˮ�ϰ������������䣬�����μӣ����������ܽ⣬Al��OH��3+OH-�TAlO2-+2H2O����A��ȷ��B��ʯ��ˮ��ͨ�������̼���ȷ���Ca��OH��2+CO2�TCaCO3��+H2O�����ɳ���������CO2+CaCO3+H2O�TCa��HCO3��2�����Ȳ���������������ܽ⣬ǰ�������ֶ�����̼�����ʵ���Ϊ1��1����B��ȷ��C����NaOH��Na2CO3�Ļ��Һ�еμ����ᣬ�����Ⱥ��������Ʒ�Ӧ����ʼû�г�����Ȼ������̼���Ʒ�Ӧ��Na2CO3+HCl=NaHCO3+NaCl��NaHCO3++HCl=NaCl+CO2��+H2O������CO2���壬��ʼδ�����������ĵ�����Ӧ�Ȳ����������ĵ�����࣬ͼ�����C����D����������Һ����μ���Ba��OH��2��Һ����Al3+ǡ��ȫ������ʱ�����ӷ���ʽΪ��2Al3++3SO42-+3Ba2++6OH-=3BaSO4��+2Al��OH��3���������μӣ�����Al3++4OH-�TAlO2-+2H2O����D��ȷ����ѡC��

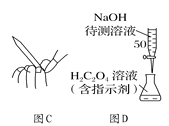
����Ŀ�������ʵ�������������������ʵ��Ӧ�ã������±���������һ���б����ĵ�����������գ�
ʵ�� | ���� |
(1)���������� | ���� |
(2)��������Ũ������ | |
(3)��ʳ���ؽ������ӣ����������ȴ�����ţ�������ⶾ�� | |
(4)��������Һ�м����������͵��������Һ���ֳ��� | |
(5))���þƾ���ϴ�˿� | |
(6)����ʳ�� |
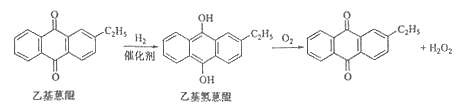
����Ŀ����ѧ����ѡ��2����ѧ�뼼��]˫��ˮ��һ����Ҫ����������Ư����������������˫��ˮ���������������䷴Ӧԭ��������������ͼ��ʾ��
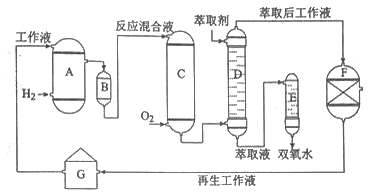
A���⻯�� |
B�������� |
C�������� |
D����ȡ�� |
E��������
F������Һ����װ��
G������Һ����װ�� ���������У����һ����������л��ܼ����Ƴɹ���Һ����һ�����¶ȡ�ѹ���ʹ��������½����⻯���پ���������ȡ�������ȹ��յõ�˫��ˮ���ش��������⣺
��1���������Ʊ�˫��ˮ���������ĵ�ԭ���� ��ѭ��ʹ�õ�ԭ���� �����ƹ���Һʱ�����л��ܼ���������ˮ��ԭ���� ��
��2���⻯��A�з�Ӧ�Ļ�ѧ����ʽΪ ������������C�ķ�Ӧ���Һ�е���Ҫ����Ϊ ��
��3����ȡ��D�е���ȡ���ǣ�ѡ��������ȡ����ԭ���� ��
��4������Һ����װ��F��Ҫ����������H2O2��ԭ���� ��
��5��˫��ˮŨ�ȿ��������������� KMnO4��Һ�ⶨ���÷�Ӧ�����ӷ���ʽΪ___________һ��˫��ˮ����������Ϊ27.5%�����ܶ�Ϊ1.10g��cm3������Ũ��Ϊ mol/L��