��Ŀ����
15������ͼ����ʯī���缫�ĵ����У��׳���Ϊ500mL��ijһ���ʵ���ɫ��Һ���ҳ���Ϊ500mLϡ���ᣬ�պ�K1���Ͽ�K2���е�⣬�۲쵽A�缫�����к�ɫ�Ĺ�̬�������ɣ�B�缫����ɫ�������ɣ�����Һ�е�ԭ��������ȫ��������ֹͣ��⣬ȡ��A�缫��ϴ�ӡ�����������缫��������1.6g����ش��������⣺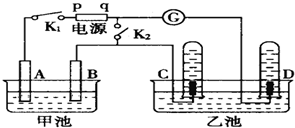
��1���������У��ҳ�C�缫������Ӧ�ĵ缫��Ӧʽ2H++2e-=H2����
��2���׳ص��ʱ��Ӧ�����ӷ���ʽ2Cu2++2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2Cu+O2��+4H+��
��3���׳ص�����Һ��pH=1��Ҫʹ������Һ�ָ������ǰ��״̬���������CuO��������Ϊ2g����������ǰ����Һ��������䣩
��4���������ٽ�K1�Ͽ����պ�K2��������ָ�뷢��ƫת����D�缫������Ӧ�ĵ缫��ӦʽO2+4e-+4H+=2H2O��
���� ��1��A�缫�����к�ɫ�Ĺ�̬�������ɣ�����A�缫��������B�缫����ɫ�������ɣ�����B������������C���������õ缫������������������D�缫���������ü��ϲ�����������������q��������p�Ǹ��������ݵ缫�����ӵķŵ������д�缫��Ӧʽ��
��2���������ͭ�������ᡢͭ���������ݴ˻ش�
��3���������ɵ�ͭ�����������������������������pH������ʸ�ԭ�ķ�������ʲô��ʲô��
��4���ٽ�K1�Ͽ����պ�K2��������ָ�뷢��ƫת���γ�����ȼ�ϵ�أ�ͨ�������������������õ��ӵĻ�ԭ��Ӧ��
��� �⣺A�缫�����к�ɫ�Ĺ�̬�������ɣ�����A�缫��������B�缫����ɫ�������ɣ�����B������������C���������õ缫������������������D�缫���������ü��ϲ�����������������q��������p�Ǹ�����
��1���������У��ҳ�C�缫���������õ缫������Ӧ�ĵ缫��ӦʽΪ��2H++2e-=H2�����ʴ�Ϊ��2H++2e-=H2����
��2���������ͭ�������ᡢͭ���������׳ص��ʱ��Ӧ�����ӷ���ʽΪ��2Cu2++2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2Cu+O2��+4H+��
�ʴ�Ϊ��2Cu2++2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2Cu+O2��+4H+��
��3��ȡ��A�缫��ϴ�ӡ�����������缫��������1.6g�������ɽ���ͭ�����ʵ�����0.025mol������2Cu2++2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2Cu+O2��+4H+��
���������������ӵ����ʵ�����0.05mol������������Ũ����$\frac{0.05mol}{0.5L}$=0.1mol/L��pH=1������ʸ�ԭ�ķ�������ʲô��ʲô��Ҫʹ������Һ�ָ������ǰ��״̬������ӣ�0.025mol������ͭ��������2g���ʴ�Ϊ��1��CuO��2��
��4���ٽ�K1�Ͽ����պ�K2��������ָ�뷢��ƫת���γ�����ȼ�ϵ�أ�ͨ���������������õ缫�Ϸ����õ��ӵĻ�ԭ��Ӧ��O2+4e-+4H+=2H2O��
�ʴ�Ϊ��O2+4e-+4H+=2H2O��
���� �����ۺϿ���ѧ��ԭ��غ͵��صĹ���ԭ���Լ��缫��Ӧʽ����д�ͼ���֪ʶ�������ۺ�֪ʶ�Ŀ��飬֪ʶ�Ĺ��ɺ������ǹؼ����ѶȲ���

 ��ʦ����ָ���ο�ʱϵ�д�
��ʦ����ָ���ο�ʱϵ�д�| A�� | pH=1����Һ�У�Na+��ClO?��SO42?��I? | |
| B�� | c��OH-��=1��10-13mol/L����Һ�У�Mg2+��Cu2+��SO42-��NO3- | |
| C�� | ������Һ�У�Fe3+��K+��Cl?��SO42? | |
| D�� | ��ɫ��Һ�У�Fe2+��NH4+��SCN-��SO42- |
| A�� | 10% | B�� | 22% | C�� | 19.6% | D�� | 28% |
| A�� | CH4 C2H4 | B�� | CH4 C3H6 | C�� | C2H4 C3H4 | D�� | C2H4 CH4 |
�ٺ��ܡ��ڲ�ݡ���ú̿����̫���ܡ�����������Һ��ʯ��������ˮú��������Ȼ����
| A�� | �ڢۢޢ� | B�� | �٢ܢ� | C�� | �ۢޢߢ� | D�� | �٢ڢ� |
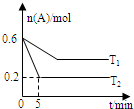 һ�������£���ӦA��g��?B��g��+C��g�� ��2.0L���ܱ������н��У���T1��T2�����¶���A�����ʵ�����ʱ��Ĺ�ϵ��ͼ��ʾ�������й�˵����ȷ���ǣ�������
һ�������£���ӦA��g��?B��g��+C��g�� ��2.0L���ܱ������н��У���T1��T2�����¶���A�����ʵ�����ʱ��Ĺ�ϵ��ͼ��ʾ�������й�˵����ȷ���ǣ�������| A�� | ����Ӧ��H��0 | |
| B�� | ƽ�ⳣ��K��T2����K��T1�� | |
| C�� | T2ʱ���ﵽƽ��ʱA��ת����Ϊ33.3% | |
| D�� | T2ʱ���÷�Ӧ�Ļ�ѧƽ�ⳣ��Ϊ0.8 |
 ԭ�����ֱ�Ӱѻ�ѧ��ת��Ϊ���ܵ�װ�ã�
ԭ�����ֱ�Ӱѻ�ѧ��ת��Ϊ���ܵ�װ�ã�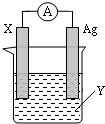 ����������ԭ��Ӧ��2Ag+��aq��+Cu��s���TCu2+��aq��+2Ag��s�� ��Ƶ�ԭ�����ͼ��ʾ����ش��������⣺
����������ԭ��Ӧ��2Ag+��aq��+Cu��s���TCu2+��aq��+2Ag��s�� ��Ƶ�ԭ�����ͼ��ʾ����ش��������⣺ ��1������ʽ��
��1������ʽ�� ��ʾ�ķ���ʽC6H14��������2-�����飻
��ʾ�ķ���ʽC6H14��������2-�����飻