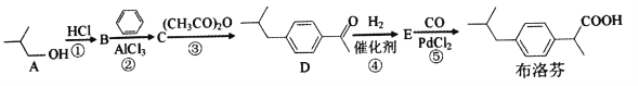
��Ŀ����
����Ŀ����1����֪����ʱ��0.1 mol/LijһԪ��HA��ˮ����0.1%�������룬�����Һ��pH��________������ĵ���ƽ�ⳣ��K��________����HA�������H����Ũ��ԼΪˮ�������H����Ũ�ȵ�________����
��2����������HA��������NaA�Ļ����Һ���ڻ�ѧ������������Һ�������м�����������ʱ����Һ������Ա仯����
�������Һ�м�����������ʱ��������Ӧ�����ӷ���ʽ��________________�������м�������KOH��Һʱ��������Ӧ�����ӷ���ʽ��________________��
���ֽ�0.02 mol��L��1 HA��Һ��0.01 mol��L��1 NaOH��Һ�������ϣ��õ�������Һ��
a����HAΪHCN������Һ�Լ��ԣ�����Һ��c(Na��)________c(CN��)(����<��������������>��)��
b����HAΪCH3COOH������Һ�����ԣ�����Һ�����е�������Ũ���ɴ�С���е�˳����____________________________��
���𰸡� 4 10��7 106 A����H��===HA HA��OH��===A����H2O > c(CH3COO��)>c(Na��)>c(H��)>c(OH��)
�������������������1������ʱ��0.1 mol/LijһԪ��HA��ˮ����0.1%������������c(H��)= 0.1 mol/L��0.1%������ƽ�ⳣ��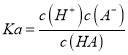 ����HA�������H����Ũ��ԼΪ
����HA�������H����Ũ��ԼΪ![]() ��ˮ�������H��Ũ��Ϊ
��ˮ�������H��Ũ��Ϊ![]() ����2���������Һ�м�����������ʱ��NaA�����ᷴӦ�����Ȼ��ƺ�HA�������м�������KOH��Һʱ��HA��KOH��Ӧ����KA��ˮ������0.02 mol��L��1 HA��Һ��0.01 mol��L��1 NaOH��Һ������������Һ�е������ǵ�Ũ�ȵ�NaA��HA������������HA�������A-ˮ�⣻���ʼ�����A-ˮ�����HA������
����2���������Һ�м�����������ʱ��NaA�����ᷴӦ�����Ȼ��ƺ�HA�������м�������KOH��Һʱ��HA��KOH��Ӧ����KA��ˮ������0.02 mol��L��1 HA��Һ��0.01 mol��L��1 NaOH��Һ������������Һ�е������ǵ�Ũ�ȵ�NaA��HA������������HA�������A-ˮ�⣻���ʼ�����A-ˮ�����HA������
��������1������ʱ��0.1 mol/LijһԪ��HA��ˮ����0.1%������������c(H��)= 0.1 mol/L��0.1%=![]() mol/L������PH=4������ƽ�ⳣ��
mol/L������PH=4������ƽ�ⳣ��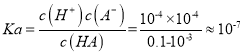 ���������ϼ��㣬��HA�������H����Ũ��ԼΪ
���������ϼ��㣬��HA�������H����Ũ��ԼΪ![]() ��ˮ�������H��Ũ��Ϊ
��ˮ�������H��Ũ��Ϊ![]() ��������HA�������H����Ũ��ԼΪˮ�������H����Ũ�ȵ�106������2���������Һ�м�����������ʱ��NaA�����ᷴӦ�����Ȼ��ƺ�HA����Ӧ���ӷ���ʽ��A����H��===HA�������м�������KOH��Һʱ��HA��KOH��Ӧ����KA��ˮ����Ӧ���ӷ���ʽ��HA��OH��===A����H2O���ڽ�0.02 mol��L��1 HA��Һ��0.01 mol��L��1 NaOH��Һ������������Һ�е������ǵ�Ũ�ȵ�NaA��HA��a����HAΪHCN������Һ�Լ��ԣ���CN��ˮ�����HCN���룬������Һ��c(Na��)>c(CN��)��b����HAΪCH3COOH������Һ�����ԣ���CH3COOH�������CH3COO��ˮ��,��Һ�����е����Ӱ�Ũ���ɴ�С���е�˳����c(CH3COO��)>c(Na��)>c(H��)>c(OH��)��
��������HA�������H����Ũ��ԼΪˮ�������H����Ũ�ȵ�106������2���������Һ�м�����������ʱ��NaA�����ᷴӦ�����Ȼ��ƺ�HA����Ӧ���ӷ���ʽ��A����H��===HA�������м�������KOH��Һʱ��HA��KOH��Ӧ����KA��ˮ����Ӧ���ӷ���ʽ��HA��OH��===A����H2O���ڽ�0.02 mol��L��1 HA��Һ��0.01 mol��L��1 NaOH��Һ������������Һ�е������ǵ�Ũ�ȵ�NaA��HA��a����HAΪHCN������Һ�Լ��ԣ���CN��ˮ�����HCN���룬������Һ��c(Na��)>c(CN��)��b����HAΪCH3COOH������Һ�����ԣ���CH3COOH�������CH3COO��ˮ��,��Һ�����е����Ӱ�Ũ���ɴ�С���е�˳����c(CH3COO��)>c(Na��)>c(H��)>c(OH��)��

����Ŀ��)����ʯ����MgO��Fe2O3��Al2O3��SiO2��ɡ�������ʯ��ȡ��ʽ̼��þʵ�鲽�����£�

��֪��
�������� | Fe(OH)3 | Al(OH)3 | Mg(OH)2 |
��ʼ����pH | 1.5 | 3.3 | 9.4 |
��1������ʯ�������ܽ����Һ�����Mg2���⣬�����е���������________________��
��2������������ʱ��������ҺpH��7��8(�й��������������pH���ϱ�)��Ca(OH)2���ܹ�������Ca(OH)2�������ܻᵼ��________�ܽ⡢________������
��3������ѭ��ʹ�ã��ܽ�Լ��Դ������ʵ���У�����ѭ��ʹ�õ�������______________��
��4���������ռ�ʽ̼��þaMgCO3��bMg(OH)2��cH2O�õ�MgO��ȡ��ʽ̼��þ4.66 g���������������أ��õ�����2.00 g�ͱ�״����CO2 0.896 L��ͨ������ȷ����ʽ̼��þ�Ļ�ѧʽΪ ____________________��(д��������̣����÷�)