��Ŀ����
ij�¶��£���0.2 mol C(s)��0.3 mol H2O(g)��Ͷ��2 L���ܱ������У�������ӦC(s)��H2O(g) CO(g)��H2(g)��5 min�ﵽƽ����ܶ�������0.3 g��L��1���й�����˵����ȷ����
CO(g)��H2(g)��5 min�ﵽƽ����ܶ�������0.3 g��L��1���й�����˵����ȷ����
 CO(g)��H2(g)��5 min�ﵽƽ����ܶ�������0.3 g��L��1���й�����˵����ȷ����
CO(g)��H2(g)��5 min�ﵽƽ����ܶ�������0.3 g��L��1���й�����˵����ȷ����| A���ӷ�Ӧ��ʼ��ƽ������У���C����ʾ�÷�Ӧ��ƽ������Ϊ0.005 mol��L��1��min��1 |
| B����ƽ��ʱѹǿ��Ϊԭ����7/6 |
| C�����¶��¸÷�Ӧ��ƽ�ⳣ��Ϊ0.005 |
| D���������¶Ⱥ�������䣬��ƽ����ϵ���ټ���0.2 mol C(s)��0.3 mol H2O(g)�����´ﵽƽ���H2O��ת���ʵ���16.7% |
BC
���������A��̼�ǹ��壬����������ʾ��Ӧ���ʣ�A����ȷ��B��5 min�ﵽƽ����ܶ�������0.3 g/L�������������������������0.6g������ݷ���ʽC(s)��H2O(g)
 CO(g)��H2(g)��֪���ӵ��������Dzμӷ�Ӧ��̼�����������ʵ�����0.6g��12g/mol��0.05mol����
CO(g)��H2(g)��֪���ӵ��������Dzμӷ�Ӧ��̼�����������ʵ�����0.6g��12g/mol��0.05mol����C(s)��H2O(g)
 CO(g)��H2(g)
CO(g)��H2(g)��ʼ����mol�� 0.3 0 0
ת������mol�� 0.05 0.05 0.05
ƽ������mol�� 0.25 0.05 0.05
��˴�ƽ��ʱѹǿ��Ϊԭ���ģ�0.25+0.05+0.05����0.3��7/6��B��ȷ��
���¶��¸÷�Ӧ��ƽ�ⳣ��Ϊ
 ��0.005��C��ȷ��
��0.005��C��ȷ���������¶Ⱥ�������䣬��ƽ����ϵ���ټ���0.2 mol C(s)��0.3 mol H2O(g)���൱��������ѹǿ��ƽ��������Ӧ�����ƶ������ˮ������ת���ʽ�����
 ��100%��16.67%��D����ȷ����ѡBC��
��100%��16.67%��D����ȷ����ѡBC��
��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
 ʵ���ò�ͬ�¶��µ�ƽ�����������±���
ʵ���ò�ͬ�¶��µ�ƽ�����������±���
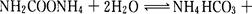
 ���о�С��ֱ������ݲ�ͬ��ʼŨ�ȵİ����������Һ�ⶨˮ�ⷴӦ���ʣ��õ�C(NH2C00-)��ʱ��ı仯������ͼ��ʾ��
���о�С��ֱ������ݲ�ͬ��ʼŨ�ȵİ����������Һ�ⶨˮ�ⷴӦ���ʣ��õ�C(NH2C00-)��ʱ��ı仯������ͼ��ʾ��
 Y(g)+Z(g) ��H��0����Ӧ��8minʱ�ﵽƽ�⣻��14minʱ�ı���ϵ���¶ȣ� 16minʱ������ƽ�⡣X�����ʵ���Ũ�ȱ仯��ͼ��ʾ�������й�˵����ȷ����
Y(g)+Z(g) ��H��0����Ӧ��8minʱ�ﵽƽ�⣻��14minʱ�ı���ϵ���¶ȣ� 16minʱ������ƽ�⡣X�����ʵ���Ũ�ȱ仯��ͼ��ʾ�������й�˵����ȷ����

 HCOOH(l) + CH3OH(l)����Ӧ���ȣ����ʱ��ֵ��С�����³�ѹ�£�ˮ�ⷴӦ���ʺ�ƽ�ⳣ������С��
HCOOH(l) + CH3OH(l)����Ӧ���ȣ����ʱ��ֵ��С�����³�ѹ�£�ˮ�ⷴӦ���ʺ�ƽ�ⳣ������С��
 M(g)��N(g)������ʵ���������±���
M(g)��N(g)������ʵ���������±���