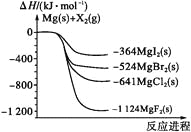
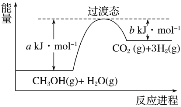
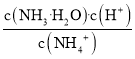��Ŀ����
����Ŀ������ȼ�տ���ͨ����������;����
;����C3H8(g)��5O2(g)===3CO2(g)��4H2O(l) ��H����a kJ��mol��1
;����C3H8(g)�D��C3H6(g)��H2(g) ��H����b kJ��mol��1
2C3H6(g)��9O2(g)===6CO2(g)��6H2O(l) ��H����c kJ��mol��1
2H2(g)��O2(g)===2H2O(l) ��H����d kJ��mol��1 (a��b��c��d��Ϊ��ֵ)
��ش��������⣺
(1)����C3H8(g)��C3H6(g)��H2(g)�ķ�Ӧ�У���Ӧ����е�������________(��������������С��������������)��������е�����������ô�ڻ�ѧ��Ӧ�У���Ӧ�����Ҫ________(�����ų�������������)��������ת��Ϊ���������䷴Ӧ������__________��
(2)��;����Ӧ��1 mol C3H8��ȫȼ��ʱ�ų�������Ϊ________ kJ(�ú�b��c��d�Ĵ���ʽ��ʾ)�������ı���ͨ������;����ȫȼ��ʱ��;����ų�������________(��������������С��������������);����ų���������
(3)a��b��c��d����ѧ��ϵʽ��________��
���𰸡�С�� ���� ���� ![]() ����
���� ![]()
��������
��1��C3H8(g)�D��C3H6(g)��H2(g) ��H����b kJ��mol��1Ϊ���ȷ�Ӧ������Ӧ���������������������������÷�Ӧ������Ҫ���ȣ�����Ҫ����������
��2����C3H8(g)�D��C3H6(g)��H2(g) ��H����b kJ��mol��1
��2C3H6(g)��9O2(g)===6CO2(g)��6H2O(l) ��H����c kJ��mol��1
��2H2(g)��O2(g)===2H2O(l) ��H����d kJ��mol��1�����ݸ�˹���ɷ������У��١�2+��+�ۣ�/2���Ȼ�ѧ����ʽC3H8(g)+ 5O2(g)= 3CO2(g)��4H2O(l) ��H��![]() kJ��mol��1�������ı���ͨ������;����ȫȼ��ʱ��Ӧ����;���أ�������;���ų���������ȡ����ݸ�˹���ɷ�������a=
kJ��mol��1�������ı���ͨ������;����ȫȼ��ʱ��Ӧ����;���أ�������;���ų���������ȡ����ݸ�˹���ɷ�������a=![]() ��
��

 ѧҵ����һ��һ��ϵ�д�
ѧҵ����һ��һ��ϵ�д� Сѧ��ʱ��ҵȫͨ����ϵ�д�
Сѧ��ʱ��ҵȫͨ����ϵ�д�����Ŀ������ʵ�顢������ؽ��۾���ȷ����
A | B | C | D | |
ʵ�� |
|
|
|
|
���� | Ʒ����ɫ | ����Թ���dz��ɫ���� | ���һ����Һʹ��̪����ɫ��Ϊ�ۺ�ɫ����30s����ԭ | ���ְ�ɫ���� |
���� | SO2 ��ǿǿ������ | �л����к�����ԭ�� | �ζ����յ� | Ksp(AgCl)��Ksp(AgI) |
A.AB.BC.CD.D