��Ŀ����
��֪A��B��Ϊ��ɫҺ�壬���з�����ζ���ڹ�ҵ�ϳ������㾫���ɷ�������ͼ��ʾ��ת��������A��B��C��E��G��Ϊ�����廯���D�ķ���ʽΪC2H4O2��E�ķ���ʽΪC7H6O2��
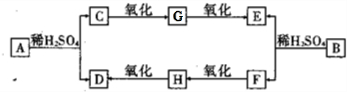
�����ͼʾ�ش��������⣺
��1��D�Ľṹ��ʽΪ ��E�Ľṹ��ʽΪ ��
��2��A��B�����ϵ���ڣ����ţ� ��
��ͬϵ���ͬ���칹���ͬһ�����ʢ�ͬһ������
��3��д������ת���Ļ�ѧ����ʽ��H��D�� ��B��E+F�� ��
��4����������3��������A��ͬ���칹�����Ŀ�� ����
i�����б����ṹ��
ii�������ϵ�һ�ȴ��������֣�
iii������NaHCO3������Ӧ�ų�CO2��
д����������һ��ͬ���칹��Ľṹ��ʽ ��

�����ͼʾ�ش��������⣺
��1��D�Ľṹ��ʽΪ
��2��A��B�����ϵ���ڣ����ţ�
��ͬϵ���ͬ���칹���ͬһ�����ʢ�ͬһ������
��3��д������ת���Ļ�ѧ����ʽ��H��D��
��4����������3��������A��ͬ���칹�����Ŀ��
i�����б����ṹ��
ii�������ϵ�һ�ȴ��������֣�
iii������NaHCO3������Ӧ�ų�CO2��
д����������һ��ͬ���칹��Ľṹ��ʽ
������A��B��Ϊ��ɫҺ�壬���з�����ζ��Ӧ����������C������������E��F������������D����C��F���к���-OH����-OH������Cԭ������2��Hԭ�ӣ�D��E�к���-COOH��D�ķ���ʽΪC2H4O2����֪DΪCH3COOH��˳�����ƿ�֪HΪCH3CHO��FΪCH3CH2OH��E�ķ���ʽΪC7H6O2������-COOH��A��B��C��E��G��Ϊ�����廯�����֪EΪ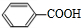 �����ƿ�֪GΪ
�����ƿ�֪GΪ ��CΪ
��CΪ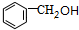 �����Ϸ�����֪AΪ
�����Ϸ�����֪AΪ ��BΪ
��BΪ ���ݴ˽��
���ݴ˽��
 �����ƿ�֪GΪ
�����ƿ�֪GΪ ��CΪ
��CΪ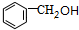 �����Ϸ�����֪AΪ
�����Ϸ�����֪AΪ ��BΪ
��BΪ ���ݴ˽��
���ݴ˽������⣺A��B��Ϊ��ɫҺ�壬���з�����ζ��Ӧ����������C������������E��F������������D����C��F���к���-OH����-OH������Cԭ������2��Hԭ�ӣ�D��E�к���-COOH��D�ķ���ʽΪC2H4O2����֪DΪCH3COOH��˳�����ƿ�֪HΪCH3CHO��FΪCH3CH2OH��E�ķ���ʽΪC7H6O2������-COOH��A��B��C��E��G��Ϊ�����廯�����֪EΪ �����ƿ�֪GΪ
�����ƿ�֪GΪ ��CΪ
��CΪ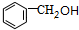 �����Ϸ�����֪AΪ
�����Ϸ�����֪AΪ ��BΪ
��BΪ ��
��
��1��������������֪��D�Ľṹ��ʽΪCH3COOH��E�Ľṹ��ʽΪ ��
��
�ʴ�Ϊ��CH3COOH�� ��
��
��2��AΪ ��BΪ
��BΪ ������Ϊͬ���칹�壬����������
������Ϊͬ���칹�壬����������
�ʴ�Ϊ���ڢܣ�
��3��H��D�Ļ�ѧ����ʽΪ��CH3CHO+2Cu��OH��2
CH3COOH+Cu2O��+2H2O����2CH3CHO+O2
2CH3COOH����
B��E+F�Ļ�ѧ����ʽΪ�� +H2O
+H2O
 +CH3CH2OH��
+CH3CH2OH��
�ʴ�Ϊ��CH3CHO+2Cu��OH��2
CH3COOH+Cu2O��+2H2O����2CH3CHO+O2
2CH3COOH���� +H2O
+H2O
 +CH3CH2OH��
+CH3CH2OH��
��4�� ��ͬ���칹���У����б����ṹ������NaHCO3������Ӧ�ų�CO2�������к���-COOH�������ϵ�һ�ȴ��������֣�������2���������ڶ�λ��Ϊ-CH3��-CH2COOH��-COOH��-CH2CH3��������������������1��-COOH��2��-CH3�������������ڼ�λ������λ���Ȼ������м䣬����������ͬ���칹�干��4�֣�����һ��Ϊ
��ͬ���칹���У����б����ṹ������NaHCO3������Ӧ�ų�CO2�������к���-COOH�������ϵ�һ�ȴ��������֣�������2���������ڶ�λ��Ϊ-CH3��-CH2COOH��-COOH��-CH2CH3��������������������1��-COOH��2��-CH3�������������ڼ�λ������λ���Ȼ������м䣬����������ͬ���칹�干��4�֣�����һ��Ϊ ��
��
�ʴ�Ϊ��4�� ��
��
 �����ƿ�֪GΪ
�����ƿ�֪GΪ ��CΪ
��CΪ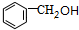 �����Ϸ�����֪AΪ
�����Ϸ�����֪AΪ ��BΪ
��BΪ ��
����1��������������֪��D�Ľṹ��ʽΪCH3COOH��E�Ľṹ��ʽΪ
 ��
���ʴ�Ϊ��CH3COOH��
 ��
����2��AΪ
 ��BΪ
��BΪ ������Ϊͬ���칹�壬����������
������Ϊͬ���칹�壬�����������ʴ�Ϊ���ڢܣ�
��3��H��D�Ļ�ѧ����ʽΪ��CH3CHO+2Cu��OH��2
| �� |
| ���� |
B��E+F�Ļ�ѧ����ʽΪ��
 +H2O
+H2O| ϡ���� |
| �� |
 +CH3CH2OH��
+CH3CH2OH���ʴ�Ϊ��CH3CHO+2Cu��OH��2
| �� |
| ���� |
 +H2O
+H2O| ϡ���� |
| �� |
 +CH3CH2OH��
+CH3CH2OH����4��
 ��ͬ���칹���У����б����ṹ������NaHCO3������Ӧ�ų�CO2�������к���-COOH�������ϵ�һ�ȴ��������֣�������2���������ڶ�λ��Ϊ-CH3��-CH2COOH��-COOH��-CH2CH3��������������������1��-COOH��2��-CH3�������������ڼ�λ������λ���Ȼ������м䣬����������ͬ���칹�干��4�֣�����һ��Ϊ
��ͬ���칹���У����б����ṹ������NaHCO3������Ӧ�ų�CO2�������к���-COOH�������ϵ�һ�ȴ��������֣�������2���������ڶ�λ��Ϊ-CH3��-CH2COOH��-COOH��-CH2CH3��������������������1��-COOH��2��-CH3�������������ڼ�λ������λ���Ȼ������м䣬����������ͬ���칹�干��4�֣�����һ��Ϊ ��
���ʴ�Ϊ��4��
 ��
�����������⿼���л�����ƶϣ��漰����ȩ�����ᡢ�������ʼ��ת���������ת������Ŀ��Ϣȷ��D��E�Ľṹʽ�ؼ����Ѷ��еȣ�

��ϰ��ϵ�д�
 ����Ӣ��ϵ�д�
����Ӣ��ϵ�д�
�����Ŀ


 ijѧϰС���ѧ���ñ�Ũ�ȵ�����������Һ�ⶨδ֪Ũ�ȵ�������Һ��
ijѧϰС���ѧ���ñ�Ũ�ȵ�����������Һ�ⶨδ֪Ũ�ȵ�������Һ��