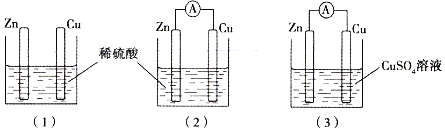
��Ŀ����
����Ŀ����100 mL FeI2��Һ����ͨ��Cl2������n(I2)��n(Fe3��)��ͨ��n(Cl2)�ı仯��ͼ��ʾ������˵������ȷ����
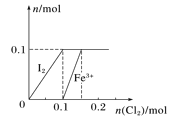
A. ��ԭ��ǿ����Fe2��<I��
B. n(Cl2)��0.12 molʱ����Һ�е�������Ҫ��Fe2����Fe3����Cl��
C. ��ͼ��֪����FeI2��Һ��Ũ��Ϊ1mol��L��l
D. n(Cl2)��n(FeI2)��1��2ʱ����Ӧ�����ӷ���ʽΪ��2Fe2����Cl2===2Fe3����2Cl��
���𰸡�D
��������
��ͼ��֪��I���ȱ�������Fe2��������������������ԭ��Ӧ����˭ǿ˭�ȷ�Ӧ����֪��ԭ��ǿ����Fe2��<I��
A.�з�����֪��ȷ��
B.��ͼ���Կ�����n(Cl2)��0.12 molʱ��I����������I2��Fe2�����ֱ�������Fe3������Һ�е�������Ҫ��Fe2����Fe3����Cl��������ȷ��
C.���ݵ�Ԫ���غ��֪n(FeI2)��0.1mol��c=n/v=1mol��L��l,����ȷ��
D. n(Cl2)��n(FeI2)��1��2ʱ����Ӧ�����ӷ���ʽΪ��2I����Cl2=== I2��2Cl�����ʴ���

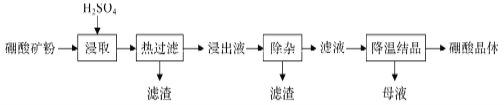
����Ŀ������þ��(2MgO��B2O3��H2O��SiO2������Fe3O4��CaCO3��A12O3)Ϊԭ����������Ĺ����������£�
��֪����һ����ͬ�¶���H3BO3���ܽ��
�¶��棩 | 20 | 40 | 60 | 100 |
�ܽ�ȣ�g�� | 5.0 | 8.7 | 14.8 | 40.2 |
��������ͬ���ʳ�����ȫʱ��pH
���� | Fe��OH��3 | Al��OH��3 | Fe��OH��2 | Mg��OH��2 |
pH | 3.2 | 5.2 | 9.7 | 12.4 |
(1)���ڿ���к�CaCO3������ȡ��ʱ���ײ���������ĭʹ���ϴӷ�Ӧ���������Ӧ��ȡ�Ĵ�ʩΪ_________________________��
(2)������Һ�������ԣ�����H3BO3��Mg2+��SO42-��������Fe2+��Fe3+��Ca2+��Al3+�����ʡ���������ʱ�������Һ�����μ�������H2O2��MgO�����Գ�ȥ����������Ϊ_______________________��H2O2������Ϊ_____________________(�����ӷ���ʽ��ʾ)��
(3)����ȡ���������ȹ�������Ŀ��Ϊ__________________________��
(4)��ĸҺ�������ڻ�������þ����֪����þ���ܽ�����¶ȱ仯��������ͼ��ʾ������Һ�ķе���ѹǿ��������ߡ�Ϊ�˴���ĸҺ���г�ֻ���MgSO4��H2O��Ӧ��ȡ�Ĵ�ʩ�ǽ���ĸҺ������Ũ����____________________________________��
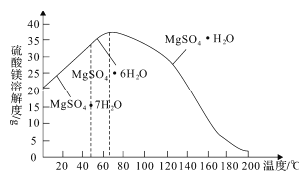
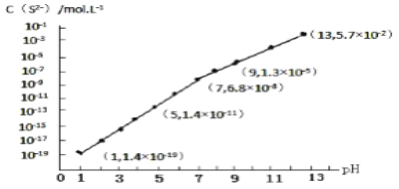
(5)��֪25��ʱ������(H3BO3)��Һ�д�������ƽ�⣺H3BO3![]() [B(OH)4]-(aq)+H+(aq)��K=5.7��10-10��25��ʱ��0.7mol��L-1������Һ��c(H+)=__________mol��L-1��
[B(OH)4]-(aq)+H+(aq)��K=5.7��10-10��25��ʱ��0.7mol��L-1������Һ��c(H+)=__________mol��L-1��
(6)��֪25��ʱ��
��ѧʽ | H2CO3 | CH3COOH |
���볣�� | K1=4.4��10-7 K2=4.7��10-11 | K=1.75��10-5 |
����˵����ȷ����________(��ѡ����ĸ)��
a��̼������Һ����������Һ���ܹ۲쵽�����ݲ���
b��̼������Һ���������Һ���ܹ۲쵽�����ݲ���
c����Ũ��̼����Һ��������Һ��pH��ǰ��>����
d����Ũ��̼������Һ�ʹ�������Һ��pH��ǰ��>����
����Ŀ��ʵ��������ͼ��ʾװ���Ʊ�����������һϵ����ص�ʵ�飨�г��豸��ʡ�ԣ���

��1��a�����������ǣ�______��
��2��ϴ��װ��B��Ϊ�˳�ȥCl2�е�HCl���壬Ӧ������Լ���_______��ͬʱװ��B���ǰ�ȫƿ�����ʵ�����ʱC���Ƿ�����������д����������ʱB�е�����___________________________��
��3��װ��C��ʵ��Ŀ������֤�����Ƿ����Ư���ԣ�Ϊ��C��I��II��III���η��루����ţ�___________��
a | b | c | d | |
I | �������ɫ���� | �������ɫ���� | ʪ�����ɫ���� | ʪ�����ɫ���� |
II | ��ʯ�� | �轺 | ��ˮ�Ȼ��� | Ũ���� |
III | ʪ�����ɫ���� | ʪ�����ɫ���� | �������ɫ���� | �������ɫ���� |
��4�����װ��D��E��Ŀ���DZȽ��ȡ��塢��ķǽ����ԡ�����D�л���ͨ��һ��������ʱ�����Կ�����ɫ��Һ��Ϊ��ɫ��˵���ǽ�������____�壨����ڡ���С�ڡ�����
��5����������װ��D��������Һ����װ��E�У����á��۲쵽��������_____________________��
��6��F��Ϊβ������װ�ã� д��ʵ���������ռ���Һ����Cl2�����ӷ���ʽ__________________________��