��Ŀ����
.������������Ҫ������������ԭ��������ˮ��Һ�ֳ�Ϊ˫��ˮ��������������ɱ����Ư�ȣ�ij��ѧ��ȤС��ȡһ�����Ĺ���������Һ��ȷ�ⶨ�˹�������ĺ�������̽���˹�����������ʣ�����д���пհף�
�ⶨ��������ĺ���
��1����ȡ10.00mL�ܶ�Ϊ�� g/mL�Ĺ���������Һ��250mL
��2���ø�����ر���Һ�ζ������������䷴Ӧ�Ļ�ѧ����ʽ���£�5H2O2+2KMnO4+6HCl=2MnCl2+2KCl+5O2��+8H2O
��3���ζ�ʱ����������ر���Һע��
��4���ظ��ζ����Σ�ƽ������c mol/L KMnO4����ҺV mL����ԭ����������Һ�й����������������Ϊ
%
%��
��̽���������������
�û�ѧС��������ṩ��ʵ���������������ʵ�飬�ֱ�֤���˹�������������ԺͲ��ȶ��ԣ���ʵ���������Լ�ֻ�й���������Һ����ˮ���⻯�ص�����Һ��ʵ����������Ʒ����ѡ����
�뽫���ǵ�ʵ�鷽����ʵ�����������±���
�ⶨ��������ĺ���
��1����ȡ10.00mL�ܶ�Ϊ�� g/mL�Ĺ���������Һ��250mL
����ƿ
����ƿ
�����������ƣ��У���ˮϡ�����̶ȣ�ҡ�ȣ���ȡϡ�ͺ�Ĺ���������Һ25.00mL����ƿ�У�����ϡ�����ữ��������ˮϡ�ͣ���������������2���ø�����ر���Һ�ζ������������䷴Ӧ�Ļ�ѧ����ʽ���£�5H2O2+2KMnO4+6HCl=2MnCl2+2KCl+5O2��+8H2O
��3���ζ�ʱ����������ر���Һע��
��ʽ
��ʽ
�����ʽ����ʽ�����ζ����У��ζ������յ������������һ�θ��������Һ����Һ�ʺ�ɫ���Ұ�����ڲ���ɫ
����һ�θ��������Һ����Һ�ʺ�ɫ���Ұ�����ڲ���ɫ
����4���ظ��ζ����Σ�ƽ������c mol/L KMnO4����ҺV mL����ԭ����������Һ�й����������������Ϊ
| 17cv |
| 2�� |
| 17cv |
| 2�� |
��̽���������������
�û�ѧС��������ṩ��ʵ���������������ʵ�飬�ֱ�֤���˹�������������ԺͲ��ȶ��ԣ���ʵ���������Լ�ֻ�й���������Һ����ˮ���⻯�ص�����Һ��ʵ����������Ʒ����ѡ����
�뽫���ǵ�ʵ�鷽����ʵ�����������±���
| ʵ �� �� �� | ʵ �� �� �� | ʵ �� �� �� |
| ��֤������ | ȡ�����⻯�ص�����Һ���Թ��У��������������Һ | ��Һ����ɫ ��Һ����ɫ |
| ��֤���ȶ��� | ȡ��������������Һ���Թ��У����ȣ��ô����ǵ�ľ������ ȡ��������������Һ���Թ��У����ȣ��ô����ǵ�ľ������ |
�������ݣ�ľ����ȼ�� |
��������1������һ�����ʵ���Ũ�ȵ���Һ��Ҫ����ƿ��
��3��������ҺҪ����ʽ�ζ�����ȡ���ζ��յ�������ǣ�����һ�θ��������Һ����Һ�ʺ�ɫ���Ұ�����ڲ���ɫ��
��4�����ݷ���ʽ�У�������غ�˫��ˮ֮��Ĺ�ϵʽ����25mL˫��ˮ��˫��ˮ���������Ӷ��ó�250mL˫��ˮ��Һ��˫��ˮ����������������������ʽ���㼴�ɣ�
II�����˫��ˮ��ʹ���۵⻯����Һ����ɫ����֤��˫��ˮ�������ԣ�
�����˫��ˮ���Ȳ�����ʹ�����ǵ�ľ����ȼ�����ʣ���֤��˫��ˮ���ȶ���
��3��������ҺҪ����ʽ�ζ�����ȡ���ζ��յ�������ǣ�����һ�θ��������Һ����Һ�ʺ�ɫ���Ұ�����ڲ���ɫ��
��4�����ݷ���ʽ�У�������غ�˫��ˮ֮��Ĺ�ϵʽ����25mL˫��ˮ��˫��ˮ���������Ӷ��ó�250mL˫��ˮ��Һ��˫��ˮ����������������������ʽ���㼴�ɣ�
II�����˫��ˮ��ʹ���۵⻯����Һ����ɫ����֤��˫��ˮ�������ԣ�
�����˫��ˮ���Ȳ�����ʹ�����ǵ�ľ����ȼ�����ʣ���֤��˫��ˮ���ȶ���
����⣺��1������һ�����ʵ���Ũ�ȵ���Һ��Ҫ����ƿ���ʴ�Ϊ������ƿ��
��3�����Ը��������Һ�����ԣ�����Ҫ����ʽ�ζ���ʢ�ţ��ζ��յ�������ǣ�����һ�θ��������Һ����Һ�ʺ�ɫ���Ұ�����ڲ���ɫ��
�ʴ�Ϊ����ʽ������һ�θ��������Һ����Һ�ʺ�ɫ���Ұ�����ڲ���ɫ��
��4����25mL˫��ˮ��Һ�к���˫��ˮ��������x��
5H2O2+6HCl+2KMnO4=2MnCl2+2KCl+5O2��+8H2O
170g 2mol
x 10-3cVmol
x=0.085 cVg
��250mL˫��ˮ��Һ��˫��ˮ��������0.85 cVg��˫��ˮ��Һ������Ϊ10�� g����������������=
��100%=
%���ʴ�Ϊ��
%��
II�����˫��ˮ��ʹ���۵⻯����Һ����ɫ����˵��˫��ˮ�������������ɵⵥ�ʣ��������۱���ɫ��������֤��˫��ˮ�������ԣ�
�����˫��ˮ���Ȳ�����ʹ�����ǵ�ľ����ȼ�����ʣ���˵��˫��ˮ�ֽ�����������������֤��˫��ˮ���ȶ���
�ʴ�Ϊ��
��3�����Ը��������Һ�����ԣ�����Ҫ����ʽ�ζ���ʢ�ţ��ζ��յ�������ǣ�����һ�θ��������Һ����Һ�ʺ�ɫ���Ұ�����ڲ���ɫ��
�ʴ�Ϊ����ʽ������һ�θ��������Һ����Һ�ʺ�ɫ���Ұ�����ڲ���ɫ��
��4����25mL˫��ˮ��Һ�к���˫��ˮ��������x��
5H2O2+6HCl+2KMnO4=2MnCl2+2KCl+5O2��+8H2O
170g 2mol
x 10-3cVmol
x=0.085 cVg
��250mL˫��ˮ��Һ��˫��ˮ��������0.85 cVg��˫��ˮ��Һ������Ϊ10�� g����������������=
| 0.85 cVg |
| 10��g |
| 17cv |
| 2�� |
| 17cv |
| 2�� |
II�����˫��ˮ��ʹ���۵⻯����Һ����ɫ����˵��˫��ˮ�������������ɵⵥ�ʣ��������۱���ɫ��������֤��˫��ˮ�������ԣ�
�����˫��ˮ���Ȳ�����ʹ�����ǵ�ľ����ȼ�����ʣ���˵��˫��ˮ�ֽ�����������������֤��˫��ˮ���ȶ���
�ʴ�Ϊ��
| ʵ������ | ʵ�鷽�� | ʵ������ |
| ��֤������ | ��Һ����ɫ | |
| ��֤���ȶ��� | ȡ��������������Һ���Թ��У����ȣ��ô����ǵ�ľ�����飮 |
���������⿼����ʵ����ƣ���ȷ���ʵ������ǽⱾ��Ĺؼ���֪���������۱���ɫ��������ʹ�����ǵ�ľ����ȼ�ֱ��ǵ����������������

��ϰ��ϵ�д�
�����Ŀ
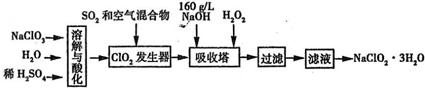



 3H2O��
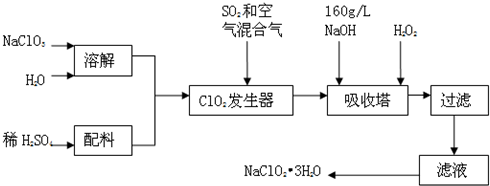
3H2O��