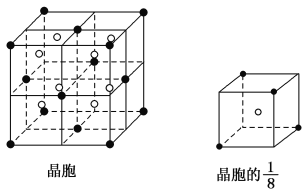
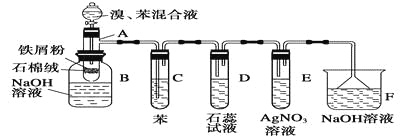
��Ŀ����
����Ŀ��ij��ɫ��Һ�к���K����Cl����OH����SO32����SO42����Ϊ������Һ��������ijЩ�����ӣ����õ��Լ��У����ᡢ���ᡢ��������Һ�����ᱵ��Һ����ˮ�ͷ�̪��Һ����������OH����ʵ�鷽��ʡ�ԣ��������������ӵĹ�������ͼ��ʾ��
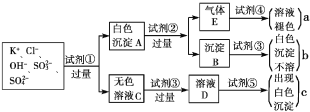
��1��ͼ���Լ��١������ʵĻ�ѧʽ�ֱ���
�� ���� ���� ���� ���� ��
��2��ͼ������a��b��c��������������ӷֱ���a ��b ��c ��
��3����ɫ����A���Լ��ڷ�Ӧ�����ӷ���ʽ��____________________________
��4����ɫ��ҺC���Լ��۵���ҪĿ����____________________________
��5����ɫ����A�����Լ��۶������Լ��ڣ���ʵ���Ӱ����____________________________
��6������Eͨ���Լ��ܷ�����Ӧ�����ӷ���ʽ��____________________________
���𰸡���1����Ba(NO3)2 ��HCl ��HNO3
��Br2 ��AgNO3 ��2��SO2��3 SO2��4 Cl��
��3��BaSO3��2H��===Ba2����SO2����H2O
��4���к�OH������ֹ��Cl���ļ����������
��5����ʹSO2��3��SO2��4�ļ���������ţ�����ȷ��SO2��4�Ƿ����
��6��SO2��Br2��2H2O===4H����SO2��4��2Br��
��������
���������ʵ��Ŀ���Ǽ�����ɫ��Һ�е������ӣ�Cl����OH����SO32-��SO42-���ݿ�ͼ�������Լ��ٽ����Ϊ���飬���Լ����ֽ�����A�����ܽ⣬���г���Bû���ܽ⣬�ݴ˿��жϳ���A�ijɷ�ΪBaSO4��BaSO3��������Eһ����SO2��������������ѡ�Լ����Լ��ٱض���Ba(NO3)2��Һ���Լ��ڱ���ϡ���ᣬ�Լ��ܱ�����ˮ����ҺC�бغ�Cl����OH����K��(ԭ��)��Ba2����NO3- (�¼���)���������ȼ��Լ��������ּ����Լ���ʱ���ְ�ɫ�����������Ƴ��Լ���ӦΪϡ���ᣬĿ�����к�OH������ֹ����һ�����Լ���AgNO3��Һ����Cl���������ţ���1��ͼ���Լ��١������ʵĻ�ѧʽ�ֱ��� ��Ba(NO3)2����HCl����HNO3����Br2����AgNO3��
��2��ͼ������a��b��c��������������ӷֱ���
A SO32-�� b SO42-�� c Cl����
��3�� ��ɫ����A���Լ��ڷ�Ӧ�����ӷ���ʽ��BaSO3��2H��===Ba2����SO2����H2O
��4����ɫ��ҺC���Լ��۵���ҪĿ�����к�OH������ֹ��Cl���ļ����������
��5����ɫ����A�����Լ��۶������Լ��ڣ���ʵ���Ӱ����
��ʹSO32����SO42���ļ���������ţ�����ȷ��SO42���Ƿ���ڡ�
��6������Eͨ���Լ��ܷ�����Ӧ�����ӷ���ʽ��SO2��Br2��2H2O===4H����SO42����2Br����

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�