��Ŀ����
��H����K����Cu2����Cl����SO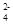 ��ѡȡ�ʵ���������ɷ�����������ĵ���ʣ����е��(������ֻ��ʹ��һ��)��
��ѡȡ�ʵ���������ɷ�����������ĵ���ʣ����е��(������ֻ��ʹ��һ��)��
(1)��̼��Ϊ�缫���е�⣬����ʵ����ʵ������٣�ˮ�����ʵ������ֲ��䣬���������������ɣ����������ͬ����õ���ʵĻ�ѧʽΪ____________������������Ӧ____________������������Ӧ________________________�������ܷ���ʽ��______________________��
(2)�Բ�˿Ϊ�缫���е�⣬ˮ�����ʵ������٣�����ʵ����ʵ������ֲ��䣬���������������ɣ����������Ϊ2��1����õ���ʵĻ�ѧʽΪ____________________��������ӦʽΪ____________________��������ӦʽΪ___________________________��
(3)���Ե�⣬����ʵ����ʵ������٣�ˮ�����ʵ���Ҳ���٣�pH�½��������ʵĻ�ѧʽΪ____________�������ܷ���ʽΪ__________________________________��
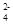 ��ѡȡ�ʵ���������ɷ�����������ĵ���ʣ����е��(������ֻ��ʹ��һ��)��
��ѡȡ�ʵ���������ɷ�����������ĵ���ʣ����е��(������ֻ��ʹ��һ��)��(1)��̼��Ϊ�缫���е�⣬����ʵ����ʵ������٣�ˮ�����ʵ������ֲ��䣬���������������ɣ����������ͬ����õ���ʵĻ�ѧʽΪ____________������������Ӧ____________������������Ӧ________________________�������ܷ���ʽ��______________________��
(2)�Բ�˿Ϊ�缫���е�⣬ˮ�����ʵ������٣�����ʵ����ʵ������ֲ��䣬���������������ɣ����������Ϊ2��1����õ���ʵĻ�ѧʽΪ____________________��������ӦʽΪ____________________��������ӦʽΪ___________________________��
(3)���Ե�⣬����ʵ����ʵ������٣�ˮ�����ʵ���Ҳ���٣�pH�½��������ʵĻ�ѧʽΪ____________�������ܷ���ʽΪ__________________________________��
10�֣�ÿ��1�֣����һ��2�֣�
(1)HCl����2H����2e����H2�� ��2Cl����2e����Cl2������ 2HCl H2����Cl2��
H2����Cl2��
(2)K2SO4����4H����4e����2H2�� ��4OH����4e����2H2O��O2��
(3)CuSO4��2CuSO4��2H2O 2Cu��O2����2H2SO4
2Cu��O2����2H2SO4
(1)HCl����2H����2e����H2�� ��2Cl����2e����Cl2������ 2HCl
 H2����Cl2��
H2����Cl2��(2)K2SO4����4H����4e����2H2�� ��4OH����4e����2H2O��O2��
(3)CuSO4��2CuSO4��2H2O
 2Cu��O2����2H2SO4
2Cu��O2����2H2SO4��������������ӷŵ�˳��ΪCu2+��Na+��H+�������ӷŵ�˳��ΪCl-��OH-��SO42-��
(1) ����ʵ����ʵ������٣�ˮ�����ʵ������ֲ��䣬���������������ɣ����������ͬ��˵������HCl��ͭ���ӡ������ӷŵ磬������������С��ˮ�����䣬�ʵ���ʵĻ�ѧʽΪHCl������������ӦΪ2H����2e����H2��������������ӦΪ2Cl����2e����Cl2���������ܷ���ʽ��2HCl
 H2����Cl2����
H2����Cl2����(2) �Բ�˿Ϊ�缫���е�⣬ˮ�����ʵ������٣�����ʵ����ʵ������ֲ��䣬���������������ɣ����������Ϊ2��1��˵��������)K2SO4�������ӡ����������ӷŵ磬�������������䣬ˮ�����٣��ʸõ���ʵĻ�ѧʽΪK2SO4��������ӦʽΪ4H����4e����2H2����������ӦʽΪ4OH����4e����2H2O��O2����
(3)���Ե�⣬����ʵ����ʵ������٣�ˮ�����ʵ���Ҳ���٣�pH�½���˵��������CuSO4����ͭ���ӡ����������ӣ��������ӡ������ӣ��ŵ磬���Ե���ʺ�ˮ�������٣��ʵ���ʵĻ�ѧʽΪCuSO4�������ܷ���ʽΪ2CuSO4��2H2O
 2Cu��O2����2H2SO4��
2Cu��O2����2H2SO4�����������⿼����ԭ������ȷ���ӵķŵ�˳���ǽ����Ĺؼ���ע��缫�����뷢���ĵ缫��Ӧ���ɽ����Ŀ�Ѷȴ�

��ϰ��ϵ�д�
�����Ŀ
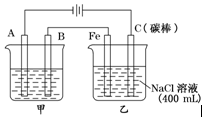

 Cd + 2NiO(OH)+ 2H2O
Cd + 2NiO(OH)+ 2H2O 