题目内容
【题目】下表为长式周期表的一部分,其中的编号代表对应的元素。
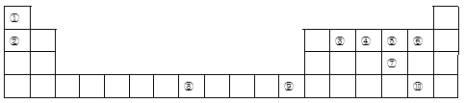
(1)比较①和②具有相同核外电子排布的简单离子半径大小关系为______________(填化学式);③、④、⑤三种元素的第一电离能由大到小的顺序为____________(填元素符号),写出原子序数和元素⑧相差2且元素⑧同族的元素基态原子的外围电子排布式____________。
(2)在元素③与①形成的原子个数比为1:1的四原子分子中,③原子的杂化方式为____________。其分子中δ键和π键数目之比为____________。
(3)元素④的某种氢化物甲分子中含有18个电子,甲为二元弱碱,在水中的电离方程式与氨相似。写出甲在水中的第一步电离的电离方程式____________。甲在微电子工业中,可作刻蚀剂H2O2的清除剂,二者发生反应的产物不污染环境,其化学方程式为____________。
(4)元素⑥和⑤形成分子乙的结构与元素①和⑤形成的最简单分子丙相似,乙的VSEPR模型为___________,解释丙的沸点高于乙的原因____________。
(5)可用赤血盐K3[Fe(CN)6]检验元素⑧的+2价阳离子,写出该反应的离子方程式___________,元素⑩的阴离子与元素⑧的+2价离子形成化合物丁,将3molCl2通入含4mol丁的溶液,用一个离子方程式表示该反应过程为____________。
(6)晶胞有两个基本要素:
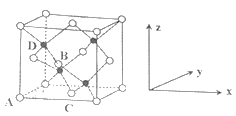
①原子坐标参数,表示晶胞内部各原子的相对位置,下图为⑦和⑨组成的离子化合物戊的晶胞,其中三个离子(白色球)坐标参数A为(0,0,0);B为(,0,);C为(,,0)。则D离子(黑色球)的坐标参数为_____。
②晶胞参数,描述晶胞的大小和形状,已知戊的晶胞参数apm,则晶胞中B和D离子的距离为______pm。
【答案】(1)H->Li+ N>O>C 3d84s2 (2)sp 3:2
(3)N2H4+H2O![]() N2H5++OH- N2H4+2H2O2=N2+4H2O
N2H5++OH- N2H4+2H2O2=N2+4H2O
(4)四面体 OF2和H2O均由分子构成,H2O分子间可形成氢键,使分子间作用强于OF2分子间的范德华力
(5)3Fe2++2[Fe(CN)6]3- = Fe3[Fe(CN)6]2↓ 3Cl2+4Fe2++2Br- =4Fe3++Br2+6Cl-
(6)①(,,) ③![]()
【解析】
试题分析:结合元素周期表可知:①为H元素、②为Li元素、③为C元素、④为N元素、⑤为O元素、⑥为F元素、⑦为S元素、⑧为Fe元素、⑨为Zn元素、⑩为Br元素;
(1)离子结构相同时核电荷数越多,离子半径越小,则离子半径H->Li+;C、N、O三种元素中因N原子2p轨道为半充满结构第一电离能相对较大,三者第一电离能由大到小的顺序为N>O>C,Mn原子序数和Fe元素相差2且和Fe元素同族,其核电荷数为28,基态外围电子排布式为3d84s2。
(2)在C元素与H元素形成的原子个数比为1:1的四原子C2H2分子中,C原子的杂化方式为sp。其分子中δ键为3,π键数目为2,两者比值为3:2。
(3)N元素的氢化物N2H4分子中含有18个电子,其为二元弱碱,水中的第一步电离的电离方程式为N2H4+H2O![]() N2H5++OH- ;N2H4还原H2O2发生反应的化学方程式为N2H4+2H2O2=N2+4H2O。
N2H5++OH- ;N2H4还原H2O2发生反应的化学方程式为N2H4+2H2O2=N2+4H2O。
(4)元素F和O形成OF2分子的结构与H2O分子相似,OF2的VSEPR模型为四面体,OF2和H2O均由分子构成,H2O分子间可形成氢键,使分子间作用强于OF2分子间的范德华力,故H2O的沸点明显高于OF2。
(5)利用赤血盐K3[Fe(CN)6]检验Fe2+离子时有蓝色沉淀生成,发生反应的离子方程式为3Fe2++2[Fe(CN)6]3- = Fe3[Fe(CN)6]2↓,将3molCl2通入含4molFeBr2的溶液,根据电子守恒,发生反应的总离子方程式为3Cl2+4Fe2++2Br- =4Fe3++Br2+6Cl-;
(6)①D与周围4个原子形成正四面体结构,D与相邻顶点的连线处于晶胞体对角线上,过面心B、C及上底面面心原子的平面且平行侧面将晶胞2等分,同理过D原子的且平衡侧面的平面将半个晶胞再2等份,可知D处于到各个面的处,则D原子的坐标参数为(,,);
②已知戊的晶胞参数apm,则晶胞中B和D离子的距离为立方体晶胞对角线的,其晶胞对角线的距离为![]() apm,则晶胞中B和D离子的距离为
apm,则晶胞中B和D离子的距离为![]() pm。
pm。

 教材全解字词句篇系列答案
教材全解字词句篇系列答案