��Ŀ����
������Ԫ��A��B��C��D�����ǵ�ԭ������������������A��C��B��D�ֱ���ͬ����Ԫ�ء���֪B��D��Ԫ�ص�ԭ�Ӻ���������֮����A��C��Ԫ��ԭ�Ӻ����������͵�2����������Ԫ�صĵ��������������塢���ֹ��塣��1��DԪ�������ڱ��е�λ����_______________����_____________�塣?
��2��д�����־�����A��B��C��D����Ԫ�صĻ��������Ӧ�ݳ���������ӷ���ʽ��
��3����AԪ�صĵ��ʺ�BԪ�صĵ��ʿ����Ƴɵ�أ������װ��ŨKOH��Һ���ö�Ľ������Ե缫����KOH��Һ�У������������Ƶ��������Ը�Ĥ����һ��ͨ��A�ĵ��ʣ���һ��ͨ��B�ĵ��ʣ���ͨ��A���ʵ�һ���ĵ缫��Ӧ����ʽ��________________��
��4��A��C��ɵĻ��������ΪҰ�⿼���ȡ�⣨H2��������д���û�����ĵ���ʽ_________���û������м���������ˮ�������������������о����֣��û�����������ijЩ���ʣ��磺Si��Al�����ټ�ˮ������Բ����������������ȡ2 mol �û�������������ĵ��ʹ��ټ���������ˮǡ�÷�Ӧ����������________________mol ��
��1����3����A?
��2��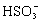 +H +�T�TSO2��+H2O
+H +�T�TSO2��+H2O
��3��H2+2OH --2e -�T�T2H2O
��4��Na +��H�ã�-
������������֪����Ԫ��λ��ӦΪ![]() ����������֮��Ϊ2������֪AΪH��BΪO��CΪNa��DΪS���ݳ�����ֻ��ΪSO2��?
����������֮��Ϊ2������֪AΪH��BΪO��CΪNa��DΪS���ݳ�����ֻ��ΪSO2��?
��3��Ϊ����ȼ�ϵ����H2��Ӧ��H2+2OH --2e -�T�T2H2O?
��4)ΪNaH������HΪ�����ӣۡ�H��-��
��5��NaH+H2O �T�T NaOH + H2��?
2 mol 2 mol 2 mol?
Si+ 2NaOH+H2O�T�TNa2SiO3+2H2��?
2 mol 2 mol

 ��У����ϵ�д�
��У����ϵ�д�