��Ŀ����
(14 ��) һ����̼���㷺Ӧ����ұ��ҵ�͵��ӹ�ҵ��
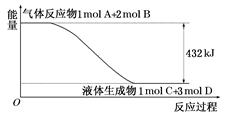
�Ÿ�¯��������Ϊ�ձ��������������ط�Ӧ���Ȼ�ѧ����ʽ���£�
4CO(g)��Fe3O4(s)��4CO2(g)��3Fe(s) ��H="a" kJ��mol��1
CO(g)��3Fe2O3(s)��CO2(g)��2Fe3O4(s) ��H="b" kJ��mol��1
��Ӧ3CO(g)��Fe2O3(s)��3CO2(g)��2Fe(s)�ġ�H= kJ��mol��1(�ú�a��b �Ĵ���ʽ��ʾ)��
�Ƶ��ӹ�ҵ��ʹ�õ�һ����̼���Լ״�Ϊԭ��ͨ�����⡢�ֽ�������Ӧ�õ���
��һ����2CH3OH(g) HCOOCH3(g)+2H2(g) ��H>0
HCOOCH3(g)+2H2(g) ��H>0
�ڶ�����HCOOCH3(g) CH3OH(g) +CO(g) ��H>0
CH3OH(g) +CO(g) ��H>0
�ٵ�һ����Ӧ�Ļ�����������ͼ��ʾ��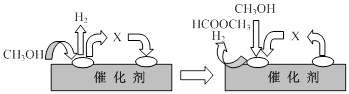
ͼ���м����X�Ľṹ��ʽΪ ��
���ڹ�ҵ�����У�Ϊ���CO�IJ��ʣ��ɲ�ȡ�ĺ�����ʩ�� ��
��Ϊ��������о�����CO��ԭ��������ʯ����Ӧ��������ʵ�X������������ͼ��ͼ��ʾ��X����������������ж�ij��̬�����Ƿ���ڣ���ͬ��̬���ʳ�������������Dz�ͬ������Ӧ�������е�һ�ֲ����������ᷴӦ���������Σ��÷�Ӧ�����ӷ���ʽΪ ��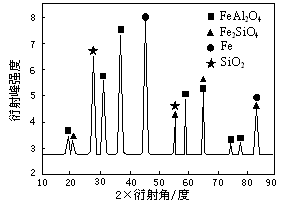
��ij������Ʒ����Ni2O340%������ΪSiO2��ͨ����ԭ���ᴿ������������ʣ�������CO��33.2 g��Ʒ�ڼ��������»�ԭΪ������Ȼ���ڳ�����ʹ�����е�Ni��CO��ϳ�Ni(CO)4���е�43 �棩������180 ��ʱʹNi(CO)4���·ֽ���������ʡ�
��������������CO�����ʵ���֮��Ϊ ��
��Ϊ��ȫ�������ҵ��������Կ����е�CO���м�⡣
�ٷۺ�ɫ��PdCl2��Һ���Լ��������������CO���������к�CO������Һ�л������ɫ��Pd������ÿ����5.3gPd��������Ӧת�Ƶ�����Ϊ ��
��ʹ�õ绯ѧһ����̼���崫����������������CO��������ṹ��ͼ��ʾ�����ִ���������ԭ���ԭ������õ�صĸ�����ӦʽΪ ��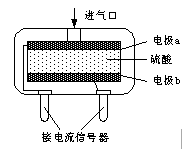
��(2a+b)/3 �Ƣ�HCHO �������¶ȣ�����ѹǿ
��FeAl2O4+8H+��Fe2++2Al3++4H2O ��3:8
�ɢ�0.1mol����0.1NA�� ��CO+H2O��2e����CO2+2H+��ÿ��2�֣���14�֣�
��������������Ž����١�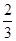 +�ڡ�
+�ڡ�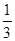 ���ܵø÷���ʽ�����ݸ�˹���ɿɸ÷�Ӧ�ȡ�H��(2a+b)/3 kJ��mol��1��
���ܵø÷���ʽ�����ݸ�˹���ɿɸ÷�Ӧ�ȡ�H��(2a+b)/3 kJ��mol��1��
�Ƣ�CH3OH���������HCHO��
�ڸ��ݵ�һ���͵ڶ����ķ�Ӧ�ص㣬��֪�������¶ȣ�����ѹǿ�������CO�IJ��ʣ�
�Ǹ��ݡ���Ӧ�������е�һ�ֲ����������ᷴӦ���������Ρ�˵���÷�Ӧ�ķ�Ӧ����FeAl2O4�����ᣬ��������FeCl2��AlCl3��H2O���ݴ˱��д�����ӷ���ʽ��
����ȷ��������������Ӧ��3CO+Ni2O3��3CO2+2Ni��Ni+4CO��Ni(CO)4����������������CO�����ʵ���֮��Ϊ3��8��
�ɢٸ��ݡ�PdCl2��Pd��2e-������ÿ����5.3gPd��������Ӧת�Ƶ�����Ϊ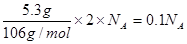 ��
��
��CO�ڸ����Ϸ���������Ӧ���ݵ�ʧ�����غ���CO��2e�D�D�DCO2���پݵ���غ���CO��2e�D�DCO2+2H+������ԭ���غ��CO+H2O��2e����CO2+2H+��
���㣺���黯ѧ��Ӧ����ԭ������Ӧ�ȵļ��㡢ƽ���ƶ�ԭ�������ӷ���ʽ����д��������ԭ��Ӧ����ת�����ļ��㡢�缫��Ӧʽ����д�ȣ���

 ��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�
��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д����б仯������������������ڷ�Ӧ�������������( )
| A��H + H = H��H | B��H��Cl =" H" + Cl |
| C��Mg + 2HCl = MgCl2 + H2 �� | D��H2SO4 + 2NaOH = Na2SO4 + 2H2O |
��14�֣�ͼa��1 mol NO2��1 mol CO��Ӧ����CO2��NO�����������仯ʾ��ͼ��ͼb�Ƿ�Ӧ�е�CO��NO��Ũ����ʱ��仯��ʾ��ͼ����������ش���������
��1��д��NO2��CO��Ӧ���Ȼ�ѧ����ʽ ��
��2���ӷ�Ӧ��ʼ��ƽ�⣬��NO2Ũ�ȱ仯��ʾƽ����Ӧ����v(NO2)�� ��
��3�����¶��¸÷�Ӧ��ƽ�ⳣ��K= ���¶Ƚ��ͣ�K ����������С�����䡱��
��4�������¶Ⱥ��ݻ���ͬ�������ܱ������У�����ͬ��ʽͶ�뷴Ӧ���÷�Ӧ�ﵽƽ�ⅼ���й��������±���
| �� �� | �� | �� | �� |
| ��Ӧ��Ͷ���� | 1 mol NO2 1 mol CO | 2 mol NO 2 mol CO2 | 1 mol NO2��1 mol CO 1 mol NO��1 mol CO2 |
| ƽ��ʱc(NO) /mol��L-1 | 1.5 | 3 | m |
| �����仯 | �ų�a kJ | ����b kJ | �ų�c kJ |
| CO��NO��ת���� | ��1 | ��2 | ��3 |
��1+��2= �� a+b/2= ,m=
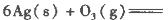
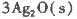 ��Ӧ����3 mol Ag2O(s)ʱ�ų�������Ϊ
��Ӧ����3 mol Ag2O(s)ʱ�ų�������Ϊ
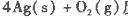 ��Ӧ����4 mol Ag(s)ʱ���յ�����Ϊ62��2 kJ���Ը�����������ж�O3ת��ΪO2��________������ȡ������ȡ�����Ӧ��
��Ӧ����4 mol Ag(s)ʱ���յ�����Ϊ62��2 kJ���Ը�����������ж�O3ת��ΪO2��________������ȡ������ȡ�����Ӧ��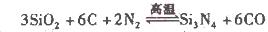
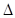 H
H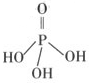
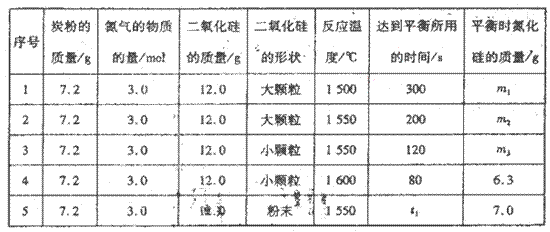
 ���ڴ����д������ƽ��Ӧʽ��a����
���ڴ����д������ƽ��Ӧʽ��a����