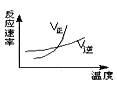
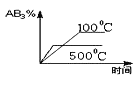
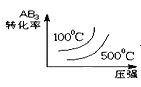
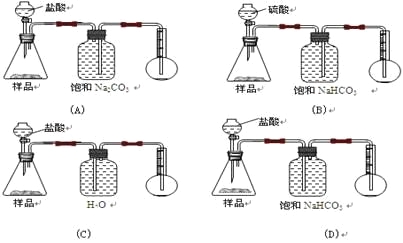
题目内容
【题目】下列各题中的物质均由核电荷数为1~10的元素组成。请按下列要求填写化学式:
(1)只由2个原子核和2个电子构成的分子是___。
(2)1个最外层有5个电子和3个只有1个电子的原子结合的分子是___。
(3)1个最外层有4个电子的原子和2个最外层有6个电子的原子结合的分子是___。
(4)由3个最外层是6个电子的原子结合而形成的分子是___。
(5)由2个原子核10个电子结合而成的分子是___。
(6)由5个原子核10个电子结合而成的分子是___。
【答案】H2 NH3 CO2 O3 HF CH4
【解析】
根据前10号元素的电子排布,由成键特点和形成分子的原子数目推导常见的分子微粒;根据原子符号的含义解题。
![]() 由题意可知,一个原子中含有一个电子,则原子为H,构成的分子为:
由题意可知,一个原子中含有一个电子,则原子为H,构成的分子为:![]() ;
;
![]() 由核外电子排布和形成分子的原子数目可知,原子分别是N和H,形成的分子为
由核外电子排布和形成分子的原子数目可知,原子分别是N和H,形成的分子为![]() ;
;
![]() 由由核外电子排布和形成分子的原子数目可知,原子分别是C和O,形成的分子为
由由核外电子排布和形成分子的原子数目可知,原子分别是C和O,形成的分子为![]() ;
;
![]() 由核外电子排布和形成分子的原子数目可知,原子是O,形成的分子为
由核外电子排布和形成分子的原子数目可知,原子是O,形成的分子为![]() ;
;
![]() 由核外电子排布和形成分子的原子数目可知,该分子为HF;
由核外电子排布和形成分子的原子数目可知,该分子为HF;
![]() 由核外电子排布和形成分子的原子数目可知,该分子为
由核外电子排布和形成分子的原子数目可知,该分子为![]() 。
。

练习册系列答案
相关题目