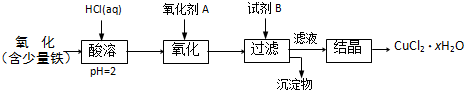
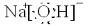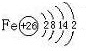��Ŀ����
��֪pHΪ4��5�������£�Cu2+������ˮ�⣬��Fe3+������ȫˮ�⡣ijѧ���õ�ⴿ����CuSO4��Һ�ķ����������ݵ缫������Cu������(m)�Լ��缫�ϲ�����������(V mL���)���ⶨCu�����ԭ���������������£�
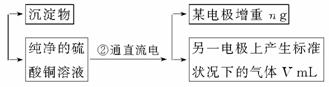
�ش��������⡣
(1)����CuO�������� _______________________________ ��
(2)����������õIJ�����������ͼ��ʾ����A��B�ֱ���ֱ����Դ�ļ�_______��_______��(���������)
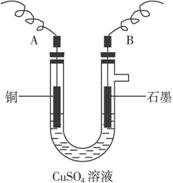
(3)��ʼ����U�ι��п��Թ۲쵽�������У�________________���������ӷ���ʽΪ________________________________________________��
(4)����ʵ������б�Ҫ����_______ (����ĸ)��
A.�������ǰ�缫������
B.���缫�ں�ɳ���ǰ������������ˮ��ϴ
C.���µ���缫��������ͭ������ϴ������
D.�����ɳ��صIJ����б��밴����ɳ����ٺ���ٳ���������
E.���п������ڵ�����£���ɵ缫�����õ��º�ɵķ���
(5)ͭ�����ԭ������Ϊ__________________ (�ô���m��V�ļ���ʽ��ʾ)��
������������һ������ʵ���ۺ��⣬������ȷ������Cu���������缫�ϲ�������������������֪��pHΪ4��5�������£�Cu2+������ˮ�⣬��Fe3+������ȫˮ�⣬���Գ�CuSO4��Һ�е�Fe(SO4)3��Һ��������������Ϣ������CuO��������H+,��ʹFe3+��ˮ��ƽ�������ƶ���ʹFe3+��ȫˮ���γ�Fe(OH)3��������ȥ�����ڱ����ǵ��CuSO4��Һ����˵缫�����Ӽ�������������ɸ��ݵ����ܻ�ѧ����ʽ��2CuSO4+2H2O![]() 2Cu+O2��+2H2SO4����Ҫȷ�ⶨ����Cu�������������ϸ���ʵ�������A��B��D��E������Dz���ȱ�ٵģ�����Cu��������O2�ڱ�״���µ���������ݷ���ʽ���ɼ����Cu�����ԭ������Ϊ
2Cu+O2��+2H2SO4����Ҫȷ�ⶨ����Cu�������������ϸ���ʵ�������A��B��D��E������Dz���ȱ�ٵģ�����Cu��������O2�ڱ�״���µ���������ݷ���ʽ���ɼ����Cu�����ԭ������Ϊ![]() ��
��
�𰸣�(1)ͨ������H+��������Һ��pHʹ֮���ߣ���Ŀ����ʹFe3+��ȫˮ���γ�Fe(OH)3��������ȥ
(2)�� ��
(3)A�����к�ɫ����������B�����������ݳ�����Һ��ɫ��dz
2Cu2++2H2O![]() 2Cu+4H++O2��
2Cu+4H++O2��
(4)A��B��D��E (5) ![]()



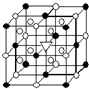 A����1����ͼ��ʾΪ����ʯ����ѧʽΪNa3AlF6���ľ�����ͼ�С�λ�ڴ������嶥������ģ���λ�ڴ��������12������е��8��С����������ģ���ͼ�С��е�һ�֣�ͼ�С�ֱ�ָ����������
A����1����ͼ��ʾΪ����ʯ����ѧʽΪNa3AlF6���ľ�����ͼ�С�λ�ڴ������嶥������ģ���λ�ڴ��������12������е��8��С����������ģ���ͼ�С��е�һ�֣�ͼ�С�ֱ�ָ����������